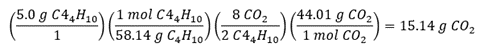Practice Test: Chemistry (67)
Answer Key, Sample Responses, Evaluation Chart, and Score Calculation Tool
Answer Key
Fill in your answers below and then print this answer key to save your work. Alternatively, you can print the answer key first to fill it out offline as you take the practice test. Note that the correct responses will be displayed on the printed answer key, so you may want to cover them until you have completed the practice test and are ready to check your answers.
When you have finished the practice test, click on "show answers" to see how well you did on each objective. In addition, use the Evaluation Chart to determine how many questions within each objective you answered correctly.
You will not receive a score for the practice test, and there is no passing score for the practice test. However, to get a sense of how well you did, use the Score Calculation Tool to better gauge your performance and degree of readiness to take an MTEL test at an operational administration.
NOTE: When you take the actual test, you will receive a score report that provides subarea-level performance, not objective-level performance. Information about test results can be found at Score Report Explanation.
| Question Number | Your Response | Correct Response |
Related Objectives and Rationale |
|---|---|---|---|
| 1 | D |
Objective 001 Response D is correct because in the Rutherford gold foil experiment, a vast majority of the alpha particles passed through the foil without being deflected. The tiny fraction deflected showed that the atom is mostly empty space, with each atom having a positively charged nucleus in its center. This model (D) is designed so that an occasional success hitting one of the suspended balls represents alpha particles interacting with the nuclei, but mostly missing the suspended balls because of all the empty space surrounding each of the balls. Response A is incorrect because "... electrons dispersed throughout a uniform positive medium..." is a description of the plum pudding model of J. J. Thomson that predates Rutherford's gold foil experiment and is not an accurate reflection of the Rutherford model. Furthermore, in Rutherford's model, the alpha particles are deflected by the positive nuclei, not by the electrons. Response B is incorrect because the electron orbitals reflect a representation of the Bohr model of the atom that was developed after the Rutherford gold foil experiment and model were developed. Furthermore, the gold foil experiment did not draw any conclusions about alpha particles—electron interactions. Response C is incorrect because the layout of the electron orbitals reflects the quantum mechanical model of the atom, which was developed after both the Rutherford gold foil experiment and after the Rutherford model and Bohr model of the atom were developed. Furthermore, the gold foil experiment did not draw any conclusions about alpha particles—electron interactions. |
|
| 2 | A |
Objective 001 Response A is correct because the Bohr model correctly shows that electrons occupy orbitals at discrete energy levels so that discrete amounts of energy are emitted or absorbed when the energy level changes. In contrast, the Rutherford model had a problem that the electrons would "spiral" into the nucleus and emit energy in the process. Response B is incorrect because both models distinguish between the nucleus and orbiting electrons. Response C is incorrect because the electrostatic attraction between electrons and protons is part of both models. Response D is incorrect because no models can identify the specific location of electrons relative to the nucleus. A specific location is implied by the Bohr model, but it is incorrect. |
|
| 3 | A |
Objective 001 Response A is correct because the quantum mechanical model fully explains the electronic structure of an atom's electrons. The model represents the spatial region where each electron is most probably located, the energy level and sublevel occupied by each electron, the magnetic spin of each electron, and the geometric shape and orientation of the atomic orbital occupied by each electron. Response B is incorrect because the Bohr planetary model does not correctly account for the electron orbitals' energy levels and shapes or the electron spin as defined by the quantum mechanical model. Response C is incorrect because the Rutherford nuclear model locates the electrons outside the nucleus, but does not explain that the electron energy levels are quantized as demonstrated by both the Bohr and quantum mechanical models, and does not account for the electron orbitals' energy levels and shapes or the electron spin as defined by the quantum mechanical model. Response D is incorrect because the plum pudding model does not separate the electrons and protons in the atom and, therefore, does not accurately represent the states of the electrons. |
|
| 4 | B |
Objective 001 Response B is correct because in this problem the average atomic mass is calculated as (23.98501417 times 78.99 + 24.98583696 times 10.00 + 25.98259292 times 11.01) divided by 100 = 24.31. Response A is incorrect because it represents the atomic mass of the most abundant isotope, not the average atomic mass. Response C is incorrect because it represents the atomic mass of the middle isotope (by mass) rounded down, instead of representing the average atomic mass. Response D is incorrect because it represents the atomic mass of the middle isotope (by mass) rounded off to 25.00, instead of representing the average atomic mass. |
|
| 5 | A |
Objective 001 Response A is correct because in this problem, the average atomic mass is calculated as (234.041 times 0.00550 + 235.044 times 0.720 + 238.051 times 99.275) divided by 100 = 238.029. Results may vary slightly due to applications of rounding. Response B is incorrect because it represents the numeric average of the three atomic masses (sum of individual masses divided by 3), not the average atomic mass. Response C is incorrect because it represents an approximation of the isotope divided by 100 (238.051 times 99.275) divided by 100 = 236.325). Results may vary slightly due to applications of rounding. Response D is incorrect because it represents the atomic mass of the most abundant isotope, instead of representing the average atomic mass. |
|
| 6 | C |
Objective 001 Response C is correct because S has electron configuration 1s 2 2s 2 2p 6 3s 2 3p 4 . All the subshells are filled, except for 3p, which has 4 electrons. By Hund's Rule, every orbital in the 3p subshell is singly occupied before any orbital is doubly occupied. Correct Response C has the correct electron configuration ( 1s 2 2s 2 2p 6 3s 2 3p 4 ) and follows Hund's Rule. Response A is incorrect because even though it would have the correct electron configuration ( 1s 2 2s 2 2p 6 3s 2 3p 4 ), it does not follow Hund's Rule. Response B is incorrect because it does not have the right number of electrons. Response D is incorrect because it does not have the right number of electrons. |
|
| 7 | D |
Objective 001 Response D is correct because Carbon has 6 total electrons. Correct Response D shows the correct number of electrons (6), and it correctly shows one of these electrons in an excited state (a 2s electron is excited to a 2p orbital). Response A is incorrect because the electron configuration shown is the ground state electron configuration for carbon. Response B is incorrect because it shows too many electrons (7 instead of 6). Response C is incorrect because it shows too few electrons (5 instead of 6). |
|
| 8 | B |
Objective 001 Response B is correct because atomic (and ionic) size is dictated by the energy level of the valence electrons and by the nuclear charge. The higher energy level of the valence electrons means a larger atom or ion. The greater nuclear charge means the smaller the atom. The four ions— A l 3 + , N 3 negative , N a + , and O 2 negative —are isoelectronic with each other, having the N e noble gas electron configuration. Since they have differing numbers of protons in the nucleus, the attractions between the electrons and the nucleus for each ion differs. The greater the nuclear charge, the stronger the attraction between the nucleus and the electrons. Among these ions, N 3 negative has the smallest nuclear charge and, therefore, weakest nucleus-electron attractions, resulting in N 3 negative being the largest of the four ions. Response A is incorrect because all atoms have the same electron configuration and, therefore, the same number of valence electrons (and N 3 negative is the largest of the ions). Response C is incorrect because all the ions have a full valence shell (and N 3 negative is the largest of the ions). Response D is incorrect because neutrons have no charge and are found in the nucleus and, therefore, do not impact atomic size (and N 3 negative is the largest of the ions). |
|
| 9 | B |
Objective 001 Response B is correct because iron is a first-row transition element with 6 electrons in an outer 3d shell and 2 electrons in an outer 4s shell. The 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 is part of the electron configuration of the inner electrons that are not the valence electrons. Response A is incorrect because iron's valence electrons are in the 3d shell (not the 4d shell, as indicated in 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 4 d 6 Response C is incorrect because the 3d shell is filled before the 4p shell. Response D is incorrect because iron does not have f electrons and also because the third atomic energy level does not contain an f subshell. |
|
| 10 | D |
Objective 002 Response D is correct because aluminum oxide is an ionic compound. The normal oxidation state of A l is +3, which is consistent with O oxidation state of minus 2 giving a neutral compound charge. Response A is incorrect because carbon tetrachloride is a covalent compound. Furthermore, the oxidation state of Cl is minus 1 , making the oxidation state of C +4 . Response B is incorrect because Mg has a +2 oxidation state, O has a minus 2 oxidation state, making S a +6 oxidation state to give a neutral compound. Response C is incorrect because N a ion is a +1 oxidation state, and S is a minus 2 oxidation state to give a neutral compound. |
|
| 11 | A |
Objective 002 Response A is correct because based on the label, the sample is a solid at room temperature, it is a metal (malleable, lustrous, and highly conductive), and it is nonreactive with water. Tin (Sn) is the only element listed that fits all these descriptions. Response B is incorrect because alkali metals, including sodium ( N a ), are reactive with water. Response C is incorrect because sulfur (S) is a nonmetal. Response D is incorrect because mercury (Hg) is a liquid at room temperature. |
|
| 12 | D |
Objective 002 Response D is correct because electronegativity is a measure of the relative attraction for shared electrons. Higher electronegativity means greater attraction for electrons and, therefore, more likely to attract electrons, which explains why nitrogen is more likely than boron to attract the electrons of another atom. Response A is incorrect because nitrogen has a larger ionization energy than boron. Furthermore, ionization energy is not a measure of attraction for an electron from another atom. Response B is incorrect because nitrogen has a smaller atomic radius than boron. Response C is incorrect because boron does not have more electron shells. |
|
| 13 | C |
Objective 002 Response C is correct because Beryllium ( B e ) is directly above magnesium (Mg) on the periodic table. Therefore, Be will be a smaller atom and will have a greater first ionization energy than Mg. Response C is the only response option that meets both these requirements. Response A is incorrect because the first ionization energy given is less than the value for Mg. Responses B and D are incorrect because the atomic radius given in each is greater than the value for Mg. |
|
| 14 | D |
Objective 002 Response D is correct because the element with a ground state electron configuration of 1 s 2 2 s 2 is a group 2 element. All group 2 elements tend to form stable ions with a +2 charge and a noble gas electron configuration. Response A is incorrect because group 2 elements do not form stable ions with a minus 2 charge. Response B is incorrect because group 2 elements do not form stable ions with a minus 1 charge. Response C is incorrect because group 2 elements do not form stable ions with a +1 charge. |
|
| 15 | A |
Objective 002 Response A is correct because electronegativity decreases going down a group (column) of the periodic table. For example, fluorine has a higher electronegativity than that of iodine. Response B is incorrect because metals are on the left of the periodic table and nonmetals are on the right. Response C is incorrect because atomic mass increases going down a periodic table group. Response D is incorrect because atomic radius increases going down a group as a result of the presence of electrons in orbitals that are farther from the nucleus. |
|
| 16 | B |
Objective 003 Response B is correct because CF4 is a covalent compound with a single central carbon atom and four fluorine atoms. The naming convention for this compound is no prefix before carbon (1 atom) and tetra prefix (meaning 4) plus fluorine with -ine ending replaced by -ide, giving carbon tetrafluoride. Response A is incorrect because it uses the naming convention for ionic compounds (no prefixes), not a covalent compound. Response C is incorrect because it uses the mono prefix for carbon, which by convention is not used for the first atom listed in a covalent compound. Response D is incorrect because it uses the convention for an ionic compound with a cation that has multiple possible charges (no prefixes and roman numeral in parentheses), whereas CF4 is a covalent compound. |
|
| 17 | C |
Objective 003 Response C is correct because the structure shown is for salicylic acid. It contains an aromatic ring, a hydroxyl group ( minus O H , or alcohol), and a carboxylic acid group ( minus C O O H ). Correct Response C correctly identifies the carboxylic acid group. Response A is incorrect because no ketone is present in the compound. Response B is incorrect because no ester is present. Response D is incorrect because no ether is present. |
|
| 18 | A |
Objective 003 Response A is correct because the chain is 4 carbons in length, thus the name butane, and the chlorine is on the second carbon. As a result, this molecule is correctly named 2-chlorobutane. If the carbons were numbered in the other direction (left to right), the molecule would be 3-chlorobutane. This name is incorrect because IUPAC convention requires that the name with the lowest number be chosen. Response B is incorrect because the chain is four carbons in length, not three. Propane is used for chains that contain three carbons. Response C is incorrect because ethane is used for chains that contain two carbons. Response D is incorrect because pentane is used for chains that contain five carbons. |
|
| 19 | A |
Objective 004 Response A is correct because the molecules listed with only bonded electron pairs are as far away from each other as possible while still bonding to the central atom and, therefore, show no deviation from electron geometries. If lone pair electrons are on the central atom, they will be closer to the central atom than the bonded electrons and, therefore, push the bonded electrons away, compressing the bond angles causing deviation from the electron geometries. For example, C H 4 has the tetrahedral geometry 109.5°, whereas N H 3 has 1 lone pair (107.8° bond angles) and H 2 O has two lone pairs (104.5° bond angle). Response B is incorrect because more lone pair electrons (not fewer) tend to decrease the bond angle. Response C is incorrect because the number of bonding electron pairs is also needed to determine the molecular geometry. Response D is incorrect because the number of both bonding and lone pair electron regions determines the bond angles. |
|
| 20 | A |
Objective 004 Response A is correct because the Lewis structure of H 2 O has 2 bonding electron regions (2 H—O bonds) and 2 lone pair electron regions around the central (O) atom. This gives it s p 3 hybridization and a "bent" electron configuration. Response B is incorrect because C O 2 has 2 double bonds giving 2 bonding electron regions and no lone pair electron regions on the central (C) atom. This gives sp hybridization and a linear molecular geometry. Response C is incorrect because N H 3 has 3 bonding electron regions (3 H—N bonds) and one lone pair electron region on the central (N) atom. This gives s p 3 hybridization and trigonal pyramidal molecular geometry. Response D is incorrect because a C H 4 Lewis structure has 4 bonding electron regions (4 C—H bonds) and no lone pair electron regions on the central (C) atom. This gives s p 3 hybridization and a tetrahedral molecular geometry. |
|
| 21 | A |
Objective 004 Response A is correct because water ( H 2 O ) and ammonia ( N H 3 ) are the only polar molecules among the choices. Since O is more electronegative than N, H 2 O is more polar than N H 3 , making H 2 O the most polar of the molecules given. Response B is incorrect because C O 2 forms a linear molecule with the Ohs on opposite ends, making it a nonpolar molecule. Response C is incorrect because, even though N H 3 is polar, the N—H bond is less polar than the O—H bond in H 2 O , making N H 3 less polar than H 2 O . Response D is incorrect because C—H bonds are generally considered nonpolar, and the C H 4 molecular geometry is tetrahedral with the carbon as the central atom, making the net dipole moment zero and, thus, a nonpolar molecule. |
|
| 22 | A |
Objective 004 Response A is correct because among the compounds listed, only magnesium sulfide (MgS) is an ionic compound. Response B is incorrect because even though silicon dioxide ( S I O 2 ) is a high–melting-point solid, it is a covalent compound, not ionic. Response C is incorrect because hydrogen chloride (HCl) is a polar covalent compound, not an ionic compound, and it is a gas at room temperature. Response D is incorrect because carbon tetrabromide ( C B R 4 ) is a nonpolar covalent compound, not an ionic compound. |
|
| 23 | B |
Objective 004 Response B is correct because the barium ion is a group 2 element ion, so it has a +2 charge and it is completely stripped of its valence electrons. Response A is incorrect because it incorrectly shows the barium ion charge as +1. Note that it also incorrectly shows chloride ion as a negative 2 charge. Response C is incorrect because it shows the barium ion with a complete octet for valence electrons, whereas the ion has only two remaining electrons that are not normally shown in the Lewis structure. Note that it also does not show the valence electrons for the chloride ions. Response D is incorrect because it incorrectly shows two valence electrons for the barium ion. It also shows the wrong number of valence electrons for the chloride ions. |
|
| 24 | D |
Objective 004 Response D is correct because all H C l O x compounds in the options contain both oxygen and chlorine. Oxygen is the central atom in these structures. Oxygen is a highly electronegative atom and will pull electron density in its direction. Chlorine is also highly electronegative. H C l O has the fewest number of electronegative atoms, and its structure is better understood if written as H O C l to reflect the fact that oxygen is the central atom. Thus, in H C l O there is only 1 highly electronegative atom pulling electron density out of the O—H bond. Response A is incorrect because H C l O 4 has the highest number of electronegative atoms pulling electron density out of the O—H bond. Oxygen is the central atom, and the structure is more correctly represented as H O C l O 3 , with 3 oxygens attached to the chlorine. Response B is incorrect because H C l O 3 has 2 more electronegative atoms than H C l O . Response C is incorrect because H C l O 2 has 1 more electronegative atom than is present in H C l O . |
|
| 25 | D |
Objective 005 Response D is correct because we know that A is a solution until cooled, but we have no information to indicate if it is an acid or not. We know that Solid B is a compound because it decomposes. Solid C may be a pure substance, but it could either be a compound or an element. Gas D is likely a pure substance because it is a decomposition product. Gas D is unreactive in air with a flame. Only Correct Response D correctly identifies the component material. Response A is incorrect because we do not know if Solution A is an acid. Response B is incorrect because we know Solid B cannot be an element (it decomposes). Response C is incorrect because even though Solid C is likely a pure substance, we do not know if it is a compound or an element. An example of this type of behavior is sodium carbonate dissolved in water. At 30°C, it forms about 28% by mass saturated, basic solution. If the solution is cooled, a sodium carbonate hydrate solid will form. Heated to around 90°C, the solid gives off the waters of hydration, leaving sodium carbonate compound. At even higher temperatures, sodium carbonate decomposes to sodium oxide and carbon dioxide. |
|
| 26 | A |
Objective 005 Response A is correct because the physical processes among the steps listed are #1 (precipitation), #2 (filtration and drying), and #4 (solvation). Responses B, C, and D are incorrect because step 5 (flammability test) and step 3 (decomposition) are chemical processes. |
|
| 27 | D |
Objective 005 Response D is correct because the last step is to identify the unknown metals ( number 2). To arrange the densities from least dense to most dense ( number 5), density calculation ( number 3) must precede number 5. Recording mass ( number 4) and determining volume ( number 6) must come before determining density ( number 3) because mass and volume are needed in order to calculate density (which equals mass divided by volume) ( number 3). Recording mass ( number 4) should be done before determining volume ( number 6) because the volume measurement requires getting the sample wet, whereas the sample must be dry to obtain a correct mass. Comparing results to known values ( number 1) comes after density calculation ( number 3) and before identifying the unknowns ( number 2). Collectively, this gives the order 4, 6, 3, 5, 1, 2 (Correct Response D). Response A is incorrect because number 3 is missing, number 4 is given twice, and number 1 is too early in the procedure. Response B is incorrect because it is not possible to sort by density ( number 5) before determining density ( number 3), comparing the calculations to known values ( number 1) cannot happen before doing the calculations ( number 3), calculating densities ( number 5) cannot happen until after mass ( number 4) and volume ( number 6) are determined, and identifying the unknown metals ( number 2) must be the last step. Response C is incorrect because number 4 and number 6 are switched, and number 5 and number 2 are switched. |
|
| 28 | D |
Objective 005 Response D is correct because the trans arrangement of hydrogen atoms in fumaric acid allows more space for the formation of hydrogen bonds between hydrogen atoms in one molecule and C O O H groups in another, absent the same kind of steric effects found in maleic acid. Response A is incorrect because dispersion forces are present in both molecules. Response B is incorrect because there is no significant difference in polarity between the two molecules. Response C is incorrect because the interactions between carboxyl groups will not cause the observed differences in total intermolecular force strength. |
|
| 29 | A |
Objective 005 Response A is correct because a solution is a homogeneous mixture. As a gas or liquid, it is also transparent. Air at sea level is a homogenous mixture mainly of oxygen and nitrogen, with other gases such as water vapor. Response B is incorrect because fog is not a solution. Instead, it can be considered a heterogeneous mixture, or a colloid of water droplets in air. Cloudiness of the fog is an indication that it is not a solution. Response C is incorrect because whipped cream is a mixture of mostly fat dispersed in water. The dispersed fat particles give the mixture its white, opaque appearance. Response D is incorrect because smoke is solid particles dispersed in air, giving its cloudy appearance. |
|
| 30 | B |
Objective 005 Response B is correct because the environmental conditions are controlled, and the comparison stated is correct and sufficient to answer the question as to which plastic degrades more quickly. Response A is incorrect because the two plastics are not compared and degradation rates are theoretical. Response C is incorrect because degradation rates provided by the manufacturer may be biased and also because the degradation rates of the two types of plastic were likely measured using different environmental conditions. Response D is incorrect because the experiment in this option involves only one type of plastic. |
|
| 31 | A |
Objective 005 Response A is correct because 2-propanol has a density very close to that of the unknown alcohol. The density of the alcohol is calculated as . 39.4 grams divided by 50.0 centimeters Further, room temperature (20°C) falls between the melting and boiling points of 2-propanol, so 2-propanol is a liquid at room temperature. Response B is incorrect because the density of cyclopentanol is different from that calculated for the unknown alcohol. Response C is incorrect because the density of cyclohexanol is different from that of the unknown alcohol and because cyclohexanol is a solid at room temperature. Response D is incorrect because, though the density of 2-methyl-2-propanol is close to that of the unknown alcohol, 2-methyl-2-propanol is a solid at room temperature; it melts at 26°C. |
|
| 32 | D |
Objective 006 Response D is correct because the reaction N A O H plus H N O 3 yields N A N O 3 plus H 2 O is an example of a base ( N a O H ) reacting with an acid ( H N O 3 ) to form a neutral salt ( N A N O 3 ) and water ( H 2 O ), which is the description of a neutralization reaction. Response A is incorrect because a redox reaction involves the change in oxidation state of the reactants, whereas the oxidation state of the components does not change in this reaction. Response B is incorrect because a combustion reaction is a reaction involving molecular oxygen ( O 2 ). No molecular oxygen is present in this reaction. Response C is incorrect because a precipitation reaction would mean that a precipitate is formed; no precipitate is formed in this reaction. |
|
| 33 | B |
Objective 006 Response B is correct because in each reaction, the vanadium is oxidized (gives up electrons) and the other element (F, O, or N) is reduced (gains electrons), which is characteristic of a redox reaction. Response A is incorrect because oxidation describes what happens to the vanadium, but does not address the entire reaction, including that the other reactant is reduced. Response C is incorrect because an electrochemical reaction is either caused by or creates an electric current; no electric current needs to be involved in this reaction. Response D is incorrect because a single replacement reaction requires that one element replace another element in a chemical reaction; in these reactions, elements are combined (synthesis reaction). |
|
| 34 | D |
Objective 006 Response D is correct because a net ionic equation shows only the components that change in the reaction. In this case, lead ( 2 ) ion ( P b 2 + ) combines with two bromide ions ( B r negative ) to form an insoluble solid ( P b B r 2 ). Response A is incorrect because the spectator ion ( N o 3 negative ) is included in the equation. Response B is incorrect because both spectator ions (K+ and N o 3 negative ) are included in the equation, and the precipitating ion lead ( 2 ) ( P b 2 + ) is left out. Response C is incorrect because both spectator ions (K+ and N o 3 negative ) are included in the equation, and both precipitating ions lead ( 2 ) ( P b 2 plus ) and bromide ( B r negative ) are left out. |
|
| 35 | D |
Objective 006 Response D is correct because in this chemical reaction, both reactants are water soluble, dispersing as the soluble ions. However, when the ions M G 2 + combine with ions O H negative , a precipitate occurs. This precipitation is the driving force for the chemical reaction. Response A is incorrect because no electrons are transferred in the reaction. Response B is incorrect because there is no acid base reaction occurring (no neutralization). Response C is incorrect because ion transfer refers to the movement of ions between two phases present from the beginning and does not necessarily require a chemical reaction. For example, ionic transfer is the movement of ions between two liquid phases, and ion exchange is movement of ions between a solution and a solid such as for water softening. In contrast, this reaction involves only one phase at the beginning, and a second phase (precipitate) forms. |
|
| 36 | A |
Objective 006 Response A is correct because the reaction between KBr and P b N O 3 2 is a double replacement reaction, so the metals swap anion partners to form the products. The products of this reaction are K N O 3 and P b Br 2 . Further, lead bromide is insoluble based on solubility rules. Response B is incorrect because it is extremely rare for compounds to form between two metals. Response C is incorrect because, although K N O 3 is a product of this reaction, all compounds containing nitrates are soluble according to solubility rules. Response D is incorrect since this compound would result from combination of two anions of like charge. |
|
| 37 | C |
Objective 007 Response C is correct because to obtain the correct answer, the coefficients for each reactant and product need to be adjusted until the total number of each element is equal on both sides of the equation and all coefficients are whole numbers. For this unbalanced equation: C 7 H 16 plus O 2 yields C O 2 plus H 2 O the recommended strategy is to balance the elements that are present in only one compound on each side, then balance the remainder. In this case, balance C and H before attempting to balance O. The left side of the equation has 7 C and 16 H. To balance C and H, place the coefficient 7 in front of C O 2 , and 8 in front of H2O, giving: C 7 H 16 plus O 2 yields 7 C O 2 plus 8 H 2 O This gives 7 carbons (C) and 16 hydrogens (H) on both sides of the equation. As set up now, the oxygen (O) has 2 on the left and 7 times 2 + 8 = 22 on the right. To balance, use the coefficient of 11 in front of the O2 on the left side of the equation (Correct Response C). This will give 22 O atoms on both sides of the equation, balancing all the elements: C 7 H 16 plus 11 O 2 yields 7 C O 2 plus 8 H 2 O Response A is incorrect because a coefficient of 3, presumably coming from adding the oxygen subscripts (2 + 1) on the right, results in 3 times 2 = 6 oxygens on the left and 22 O on the right side of the equation and, therefore, is not balanced for O. Response B is incorrect because 7 times 2 = 14 O on the left only accounts for the 14 oxygens from the 7 C O 2 on the right, but overlooks the O in H2O. Response D is incorrect because a coefficient of 15, presumably coming from adding the coefficients on the right ( 7 C O 2 plus 8 H 2 O ) results in 30 O on the left and 22 O on the right side of the equation and, therefore, is not balanced for O. |
|
| 38 | C |
Objective 007 Response C is correct because to properly solve this problem, the following information is needed: balanced combustion equation, molar mass of C 4 H 10 , and molar mass of C O 2 . Unbalanced equation: C 4 H 10 plus O 2 yields C O 2 plus H 2 O First balance C and H: C 4 H 10 plus O 2 yields 4 C O 2 plus 5 H 2 O Then balance O using a coefficient for O2 on the left side: C 4 H 10 plus 13 halves O 2 yields 4 C O 2 plus 5 H 2 O Finally, multiply all coefficients by 2 to remove the fraction: 2 C 4 H 10 plus 13 O 2 yields 8 C O 2 plus 10 H 2 O This balanced equation gives you the C O 2 to C 4 H 10 mole ratio (8 to 2) needed to calculate quantity of C O 2 produced. Calculating molar mass of C 4 H 10 : Molar mass C 4 H 10 equals 4 times MM C plus 10 times MM H equals 4 times 12.01 plus 10 times 1.01 equals 58.14 grams/mol C 4 H 10 Calculating molar mass of C O 2 : Molar Mass C O 2 equals MM C plus 2 times MM O equals 12.01 plus 2 times 16.00 equals 44.01 grams/mol C O 2 Finally, use dimensional analysis to calculate mass of C O 2 produced from 5.0 g C 4 H 10 : 5 point 0 grams C 4 H 10 times 1 mole C 4 H 10 per 58 point 1 4 grams C 4 H 10 times 8 C O 2 per 2 C 4 H 10 times 44 point 0 1 grams C O 2 per 1 mole C O 2 equals 15 point 1 4 grams C O 2 It is possible to do the same calculations after first rounding molar masses to nearest gram and get a result that matches the correct answer. Response A is incorrect because it comes from a calculating error that incorporates a factor of 10 in the denominator (such as dividing by 581.4 instead of 58.14) or a factor of 0.1 in the numerator (such as multiplying by 0.50 instead of 5.0). Response B is incorrect because it fails to correct for C O 2 to C 4 H 10 mole ratio (8 to 2). Response D is incorrect because it fails to correct for C O 2 to C 4 H 10 mole ratio (8 to 2), and it incorporates a calculating error that incorporates a factor of 10 in the numerator (such as multiplying by 50 instead of 5.0) or a factor of 0.1 in the denominator (such as dividing by 5.814 instead of 58.14). |
|
| 39 | B |
Objective 007 Response B is correct because this titration is for the neutralization reaction: N A O H plus H C L yields N A C L plus H2O Since the coefficients for N a O H and HCl are the same, the following equation can be used to calculate the molarity of HCl: m subscript 1 v subscript 1 = m subscript 2 v subscript 2 where M represents molarity, V is volume, and the subscript 1 represents N a O H and subscript 2 represents HCl. Since the volume of N a O H and HCl are in mL, no unit changes are needed. The equation can then be rearranged to solve for M sub 2 (Correct Response B) : m subscript 2 equals the product of m subscript 1 times v subscript 1divided by v subscript 2 = the product of 37.2 milli liters times 0.50 M divided by 51.0 milli liters equals 0.36 m h c l Response A is incorrect because it comes from failing to incorporate the N a O H volume (37.2 mL) into the calculation. Response C is incorrect because it comes from incorrectly switching 37.2 mL and 51.0 mL in the calculation. Response D is incorrect because it likely comes from a combination of errors such as failing to include the 0.50 M concentration for N a O H and dividing by 31.0 mL instead of 51.0 mL. |
|
| 40 | C |
Objective 007 Response C is correct because in the table, final volume 3 recorded is more than double the volumes of runs 1 and 2. If, however, the final burette reading from trial 2 is subtracted from trial 3 final burette reading, a volume of 12.63 mL is obtained, which is consistent with the final volumes for trials 1 and 2. Note that this does not necessarily mean that the initial volume for trial 3 is 15.12 mL, but it is a good indicator of the error that occurred. Response A is incorrect because misjudging the color will give a much smaller error—approximately 1 to 2 mL or less. Response B is incorrect because the relative position for reading would give parallax error of less than 1 mL. Response D is incorrect because reversing the acid and base would show up immediately with the indicator before titration began, and it would equally impact all three trials. |
|
| 41 | A |
Objective 007 Response A is correct because both sides of the chemical equation have 1 zinc atom, 2 silver atoms, 2 nitrogen atoms, and 6 oxygen atoms. Response B is incorrect because there are 5 oxygen atoms on the left and 4 oxygen atoms on the right. Response C is incorrect because there are 2 aluminum atoms on the right and 1 on the left, and 6 oxygen atoms on the left and 3 on the right. Response D is incorrect because there are 4 hydrogen atoms on the left and 2 on the right, and 6 oxygen atoms on the left and 5 on the right. |
|
| 42 | A |
Objective 007 Response A is correct because the correct answer is calculated by finding the number of moles of oxygen that will result from the decomposition of 50.0 g of hydrogen peroxide and then remembering that at standard temperature and pressure, 1 mole occupies 22.4 liters. The calculation is as follows: 50 point 0 grams H 2 O 2 times 1 moles H 2 O 2 per 34 point 0 1 grams H 2 O 2 times 1 mole O 2 per 2 moles H 2 O 2 times 22 point 4 liters O 2 per 1 mole O 2 Response B is incorrect because it is calculated by using 32.0 L as the volume of 1 mole of oxygen gas. Response C is incorrect because it is calculated by omitting the third factor in the expression (1 mole of oxygen divided by 2 moles hydrogen peroxide). Response D is incorrect because it is calculated by using 2 moles of oxygen divided by 1 mole of hydrogen peroxide in the third factor of the expression. |
|
| 43 | B |
Objective 008 Response B is correct because relative mass of each element in elastin ( C 27 H 48 N 6 O 6 ) can be evaluated by multiplying the atomic mass of each element by the number of atoms of that element in the molecule. The element with the smallest total relative mass also has the smallest percentage in the molecule: 27 C = 27 (12.01 u) = 324.27 u of C 48 H = 48 (1.01 u) = 48.48 u of H 6 N = 6 (14.01 u) = 84.06 u of N 6 O = 6 (16.00 u) = 96.00 u of O Since mass of H atoms in the molecule is the smallest (48.48 u), it is also the lowest percentage by mass of all the elements in elastin (Correct Response B). Response A is incorrect because there is more C (324.27 u) by mass than H (48.48 u) in elastin. Response C is incorrect because there is more N (84.06 u) by mass than H (48.48 u) in elastin. Response D is incorrect because there is more O (96.00 u) by mass than H (48.48 u) in elastin. |
|
| 44 | A |
Objective 008 Response A is correct because the empirical formula for the unknown iron oxide sample can be obtained by assuming the sample is 100 g and using the percent of F e (iron) and O (oxygen) as their respective masses. The number of moles can then be calculated by dividing the mass by the molar mass. The smallest whole number ratio of the moles is then used as the mole ratio of each element for the empirical formula (Correct Response A): 77.7% F e (77.7 g) means 77.7 g divided by 55.8 g/mol = 1.39 mol F e (100% minus 77.7%) O = 23.3% O (23.3 g) means 23.3 g divided by 16.00 g/mol = 1.45 mol O 1.39 to 1.45 is approximately equal to 1 to 1, meaning the empirical formula is F e O This can be verified by calculating the percent F e as 100% times 55.8 divided by the quantity 55.8 plus 16.00 = 77.7%. Response B is incorrect because a 2 to 3 mole ratio means percent F e is 100% times 2 times 55.8 divided by the quantity of 2 times 55.8 plus 3 times 16.00 = 69.9%, which is less than 77.7% reported. Response C is incorrect because a 3 to 4 mole ratio means percent F e is 100% times 3 times 55.8 divided by the quantity of 3 times 55.8 plus 4 times 16.00 = 72.3%, which is less than 77.7% reported. Response D is incorrect because a 4 to 5 mole ratio means percent F e is 100% times 4 times 55.8 divided by the quantity of 4 times 55.8 plus 5 times 16.00 = 73.6%, which is less than 77.7% reported. |
|
| 45 | C |
Objective 008 Response C is correct because the molar mass of Zr N O 3 4 is 339.2 g/mol and is calculated as follows: 91.2 plus 4 times 14.01 plus 12 times 16.00 = 339.2 g/mol, since 91.2 is the atomic mass of zirconium, 14.01 is the atomic mass of nitrogen, and 16.00 is the atomic mass of oxygen. Response A is incorrect because it was calculated as 91.2 plus 4 times 14.01 plus 7 times 16.00 . Response B is incorrect because it was calculated as 91.2 plus 14.01 plus 12 times 16.00 —that is, it does not include a coefficient of 4 in front of the mass of nitrogen. Response D is incorrect because it was calculated as 4 times the quantity 91.2 plus 14.01 plus 3 times 16.00 . |
|
| 46 | C |
Objective 008 Response C is correct because the percent by mass of oxygen in C 3 H 5 N 3 O 9 is calculated by finding the mass of oxygen in 1 mole of C 3 H 5 N 3 O 9 , divided by the formula mass of C 3 H 5 N 3 O 9 , multiplied by 100%: 100 percent times the quotient of 9 times 16.00 divided by 3 times 12.01 plus 5 times 1.01 plus 3 times 14.01 plus 9 times 16.00 Response A is incorrect and is calculated by the omission of the 9 in the numerator of this expression. Response B is incorrect and is calculated by omission of the coefficients 9, 3, and 5 from the expression. Response D is incorrect and is approximated by replacing the 9 in the numerator of this expression with a 12. |
|
| 47 | A |
Objective 008 Response A is correct because it is calculated by first assuming 100 grams of the compound. The number of grams of each element is then divided by the atomic mass of that element: 30.9 grams N A divided by 23. 0 grams, 47.7 grams C L divided by 35.5 grams, 21.5 grams O divided by 16.00 grams In every case, the ratio that results is 1.34. Dividing through by the lowest ratio (which is 1.34, since all are 1.34) results in 1 ( N a ), 1 ( C l ), and 1 (O) for the subscripts of the elements in the chemical formula. Response B is incorrect because it is calculated as 2 times 21.5 grams O divided by 16.00 grams Dividing through by 1.34 results in 1 ( N a ), 1 ( C l ), 2 (O) for the subscripts in the chemical formula. N a C l O 2 is a chemically reasonable formula (sodium chlorite). Response C is incorrect because it is calculated as 3 times 21.5 grams O divided by 16.00 grams Dividing through by 1.34 results in 1 ( N a ), 1 ( C l ), and 3 (O) for the subscripts in the chemical formula. N a C l O 3 is a chemically reasonable formula (sodium chlorate). Response D is incorrect because it is calculated as 4 times 21.5 grams O divided by 16.00 grams Dividing through by 1.34 results in 1 ( N a ), 1 ( C l ), and 4 (O) for the subscripts in the chemical formula. N a C l O 4 is a chemically reasonable formula (sodium perchlorate). |
|
| 48 | C |
Objective 008 Response C is correct because C2H4 has a molecular weight of 28.05 amu. It is calculated as 2 times 12.01 a m u plus 4 times 1.01 a m u . It also has an empirical formula of CH2, since 2 CH2 units compose C2H4. Response A is incorrect because the empirical formula of CH is CH (not CH2) and the molecular weight is 13.02 amu. Further, this is not a reasonable molecular formula since carbon could not have an octet of electrons. Response B is incorrect because the molecular weight of CH2 is 14.03 amu. Additionally, this is not a reasonable molecular formula since carbon could not have an octet of electrons. Response D is incorrect because C3H6 has a molecular weight of 42.09 amu. This formula is chemically reasonable, and this compound is called propene. |
|
| 49 | C |
Objective 009 Response C is correct because at the point that the acid (call it H A ) is completely neutralized (equivalence point), the amount of A minus present is essentially equal to the amount of H A initially present. To determine pH, [H+] is needed. This can be determined by first determining the [ O H minus ] present from the conjugate base reaction with water: . A minus plus H 2 O yields H A plus O H minus Using this to construct the conjugate base equilibrium equation: K sub b equals the concentration of H A times the concentration of O H minus divided by the concentration of A minus To simplify, assume [A minus ] is equal to the initial H A concentration after dilution with 30.0 mL water (from the titration), which gives 0.720 M. More precisely, it should be 0.720 minus X, but X is very small compared to the 0.720 M. This makes [ H A ] = [ O H minus ] = X, the unknown value to be determined. Then, K sub b can be written as: K sub b equals X squared divided by 0.720 We can also substitute K sub b equals K sub w divided by K sub a Substituting then rearranging gives X squared equals 0 point 7 2 0 times 10 the negative 14th power divided by 1 point 3 8 times 10 to the negative fourth power Since X = [ O H minus ], then taking the log of the square root of X squared gives P O H = 5.14. Since pH = 14 minus P O H , pH = 8.86 (Correct Response C). This also makes intuitive sense because complete neutralization of a weak acid with a strong base will give a pH slightly greater than 7. Response A is incorrect because 5.14 is the minus P O H value, not the pH. Furthermore, a weak acid neutralized by a strong base will give a pH slightly greater than 7. Response B is incorrect because pH 7.00 is the pH of a strong acid completely neutralized by a strong base. Response D is incorrect because it incorrectly calculates the pH as 14 minus P O H where [ O H minus ] is erroneously set equal to the initial [ H A ] (0.720 M). |
|
| 50 | D |
Objective 009 Response D is correct because the combination of chemicals is that of a weak acid and a strong base. This conclusion can be drawn because the equivalence point on the graph corresponds to a pH greater than 7. It is clear that a weak acid is being titrated with a strong base (instead of a strong base being titrated with a weak acid) because the pH at the beginning of the titration is around 3. This pH value is characteristic of solutions of weak acids. Response A is incorrect because the graph shows only one equivalence point. If multiple protons were present, the graph would have an equivalence point for each one. Response B is incorrect because the starting pH value is that of a weak acid (3). Response C is incorrect because combining a weak acid, which is the substance being titrated, with a strong acid would not produce this type of titration curve. |
|
| 51 | C |
Objective 009 Response C is correct because the relevant chemical equation is aqueous N H 3 plus liquid H 2 O yields aqueous N H 4 plus plus aqueous O H negative and the expression for Kb is given as follows: Kb = K sub b equals the concentration of N H 4 plus times the concentration of O H minus divided by the concentration of N H 3 If 1 point 8 times 10 to the negative fifth power is substituted as the value of Kb and if 0.25 M is used as the concentration of NH3 and 0.5 M is used as the concentration of NH4+, The concentration of O H minus can be calculated. Solving for The concentration of O H minus gives a value of 9.0 times 10 to the negative sixth power molar Using P O H equals the negative log of the concentration of O H minus the concentration of O H minus gives a value of 5.0 for the p O H . Using p O H + pH = 14, pH (5 + pH = 14) can be calculated. This gives pH = 9 (Correct Response C). Response A is incorrect and is calculated as the negative logarithm of 1.8 times 10 to the negative fifth power . Response B is incorrect since a value this close to 7 (neutral) is unlikely even for a weak base—like ammonia at this concentration. Response D is incorrect and is calculated using P O H equals the negative log of 0.25 (the concentration of ammonia). This pH is then calculated using the equation pH = 14 minus 0.6. |
|
| 52 | D |
Objective 009 Response D is correct because to calculate the concentration of the N a O H solution, in units of molarity, the number of moles of N a O H and the volume of N a O H are both required. The volume of N a O H is given in the problem. At the equivalence point, the number of moles of acid is equal to the number of moles of base. The number of moles of acid is equal to 0.10 mole H 2 S O 4 per liter times 0.022 liter times 2 because there are 2 moles of H+ ion produced with dissociation of 1 mole of H 2 S O 4 . The number of moles of acid, 4.4 times 10 to the negative third power is then equivalent to the number of moles of base. The final step is to divide 4.4 times 10 to the negative third power moles by 0.010 L (the volume of N a O H ). Response A is incorrect because this number is obtained by calculating the number of moles of acid as 0.10 mole H 2 S O 4 per liter times 0.022 liter divided by 4 and then dividing the result by 0.0100 L (the volume of N a O H ). Response B is incorrect and is obtained by calculating the number of moles of acid as 0.10 mole H 2 S O 4 per liter times 0.022 liter divided by 2 (dividing by 2 instead of multiplying by 2 as is needed) and then dividing the result by 0.0100 L (the volume of N a O H ). Response C is incorrect and is obtained by calculating the number of moles of acid as 0.10 mole H 2 S O 4 per liter times 0.022 liter thereby omitting the coefficient of 2 that is needed. This value (0.0022 mole) is then divided by 0.0100 L N a O H (the volume of N a O H ). |
|
| 53 | C |
Objective 009 Response C is correct because the number of grams N a C l can be calculated as . 1.75 moles of N a C l per 1 liter times 0.050 liters times 58 point 5 grams N a C l per 1 mole N a C l Molarity is moles of solute per liter of solution. Multiplying molarity by the volume gives the number of moles of N a C l , and then multiplying by the molar mass gives the number of grams of N a C l . Response A is incorrect and was calculated as 1.75 moles N a C l per 1 liter divided by 0.050 liters Response B is incorrect and was calculated by dividing 50.0 mL N a C l by 1.75. Response D is incorrect and was calculated as 1.75 moles N a C l per 1 liter times 0.050 liters |
|
| 54 | B |
Objective 009 Response B is correct because The concentration of H 3 O plus is needed in order to find pH, because pH = the negative of the log of the concentration of H 3 O plus To find The concentration of H 3 O plus , we write Aqueous H O C L yields aqeous H 3 O plus plus aqueous O C L minus . We can then use the expression for K sub a to find The concentration of H 3 O plus : K sub a = . the concentration of H 3 O plus times the concentration of O C L minus divided by the concentration of H O C l Substituting 3.5 times 10 raised to the power of negative 7 for K sub a 0.22 M for The concentration of H O C l , x for The concentration of H 3 O plus , and x for The concentration of O C l minus and then solving for x, we find that The concentration of H 3 O plus = 2.77 times 10 raised to the power of negative 4 M. This value can then be used to calculate pH pH = negative log of the quantity 2.77 times 10 raised to the power of negative 4 = 3.56). The concentration of H 3 O plus is equal to the O C l minus , since both are formed in equimolar amounts in the dissociation of H O C l . Response A is incorrect and was calculated using the value of 0.22 M for The concentration of H 3 O plus . Response C is incorrect and was calculated by dividing K sub a by 0.22 and using this as the concentration of H 3 O plus . Response D is incorrect and was calculated by equating K sub a with The concentration of H 3 O plus . |
|
| 55 | D |
Objective 009 Response D is correct because the freezing point of the SrCl2 solution will be lower than that of pure water. The depression in freezing point can be calculated by multiplying the molal freezing point depression constant for water ( K sub f ) by the molality of the solute particles in the SrCl2 solution. K sub f is 1.86°C/m, and the molality of the particles in the SrCl2 solution is 4.53 m, 3 times 0 point 1 5 1 moles S R C L 2 divided by 0 point 1 0 0 kilograms H 2 O The molar mass of SrCl2 is 158.6 g/mol, and the 3 is needed in the calculation because SrCl2 dissociates into three ions (one Sr2+ ion and two Cl negative ions) when dissolved in water. Response A is incorrect and may have been calculated incorrectly using the boiling point depression constant for water. Response B is incorrect and may have been calculated without considering the fact that SrCl2 dissociates completely in solution (i.e., if the 3 was left out of the equation). Response C is incorrect and was calculated using a coefficient of 2 instead of 3 in the calculation of the molality of particles in the SrCl2 solution. |
|
| 56 | A |
Objective 009 Response A is correct because the boiling point increases linearly with increases in the concentration of species dissolved in solution. This is an example of a colligative property. The boiling point does not depend on the identity of the substance, only the amount. Calculating the concentration of dissolved substances for each of the solutions in the options shows that Response A has the lowest boiling point because it has the lowest concentration of dissolved substances (0.67 M). Response B is incorrect and has a concentration of 1.09 M. Response C is incorrect because zinc sulfate separates into two ions when dissolved in water. Therefore, the concentration of ions for Response C is 0.99 M, which is double the value of the concentration of either zinc or sulfate ion. Response D is incorrect because ammonium chloride will separate into two ions when dissolved in water. The concentration of the ions is 2.24 M, which is twice the value of the concentration of either ammonium or chloride ion. |
|
| 57 | B |
Objective 009 Response B is correct because the first point corresponds to 5°C and 2.0 mM. The second point corresponds to 20°C and 2.0 mM. The first point is underneath the solubility curve shown in the graph, indicating that the gas is soluble under these conditions. The second point is above the solubility curve shown in the graph, indicating that the gas is insoluble under these conditions. Response A is incorrect because the gas is soluble under both sets of conditions (both points are below the curve). Response C is incorrect because the gas is soluble under both sets of conditions. Response D is incorrect because the gas is soluble under both of these conditions. |
|
| 58 | D |
Objective 010 Solid water has both fixed volumes, with molecules arranged in an ordered fashion, and molecular motions that are limited to small vibrations. Liquids also have fixed volume, but the particles can flow past each other. Response D correctly describes properties of solid molecules. Response A is incorrect because it describes properties of a gas rather than properties of a solid. Response B is incorrect because it describes the properties of a gas rather than the properties of a liquid. Response C is incorrect because it describes properties of a solid rather than properties of a liquid. |
|
| 59 | D |
Objective 010 In the temperature-time plot, phase transitions are represented by horizontal (constant temperature) segments of the curve. For all materials, the liquid-gas phase transition temperature ( t 4 to t 5 ) is higher than the solid-liquid phase transition temperature ( t 2 to t 3 ). Response D correctly identifies the higher temperature phase transition ( t 4 to t 5 ) as the liquid-gas phase transition. Response A is incorrect because t 1 to t 2 represents increasing kinetic energy of the solid, not the liquid-gas phase transition. Response B is incorrect because t 2 to t 3 represents the lower temperature solid-liquid phase transition. Response C is incorrect because t 3 to t 5 represents both the increasing kinetic energy of the liquid ( t 3 to t 4 ) and the liquid-gas phase transition ( t 4 to t 5 ). |
|
| 60 | C |
Objective 010 Response C is correct because the diagram represents the phase diagram for a pure substance going through two phase transitions, solid to liquid, and liquid to gas. The phase transitions are indicated by adding heat without a temperature change (slope of zero in the temperature/added heat diagram). Anywhere that the slope is positive is a single phase with increasing molecular kinetic energy. Solid is the lowest kinetic energy of the single phases, so it is at the lowest temperature region (Region A), and gas is the highest kinetic energy single phase, so it is at the highest temperature region (Region E), and the middle single phase region is the liquid (Region C). So, Region C represents increasing kinetic energy of a liquid (Correct Response C). The zero slope regions (plateaus) are solid-liquid phase transition (Region B) and liquid-gas phase transition (Region D). Response A is incorrect because Region E does not represent breaking molecular interactions in a gas. Instead, it represents increasing kinetic energy of the gas phase with increasing heat. Response B is incorrect because Region B does not represent increasing molecular kinetic energy of the solid. Instead, it represents the solid-liquid phase transition. Response D is incorrect because Region A does not represent breaking intermolecular interactions in a solid. Instead, it represents increasing molecular kinetic energy of the solid with increasing heat. |
|
| 61 | C |
Objective 010 Response C is correct because volume at the summit can be determined by starting with the ideal gas relationship: P sub 1 V sub 1 over T sub 1 = P sub 2 V sub 2 over T sub 2 P, T, and V are pressure, volume, and temperature, respectively. Subscript 1 represents starting conditions and subscript 2 represents conditions at the summit. Then, solving for V sub 2 gives V sub 2 = P sub 1 V sub 1 T sub 2 over P sub 2 T sub 1 = (1.00 atmospheric pressure at sea level) times (1.25 liters) times (259.11 kelvins) all over {(0.330 atmospheric pressure at sea level) times (273.00 kelvins)} = 3.59 L, which corresponds to Response C. Response A is incorrect because T sub 1 and P sub 2 are switched with T sub 2 and P sub 1 in the calculation V sub 1 P sub 2 T sub 1 over P sub 1 T sub 2 = 0.435 Response B is incorrect because of a calculation error likely from incorrectly arranged terms such as excluding P sub 2 , and placing V sub 1 in the denominator (example: P sub 1 T sub 2 over T sub 1 V sub 1 = 0.759—close to recorded value). Response D is incorrect because it switches T sub 1 and T sub 2 in the calculation for V sub 2 . |
|
| 62 | B |
Objective 010 Response B is correct because vibration occurs in the solid state, and molecules in solids, such as crystal lattices, vibrate about fixed positions. Response A is incorrect; it is a common misconception that particles do not vibrate in the solid state. Particles in the solid state will vibrate less as temperatures of absolute zero are approached. Response C is incorrect because movement of particles in the solid state is restricted as a result of interactions with other particles (for example, N a C l in a crystal lattice). Response D is incorrect because particles in the solid state vibrate in such a way that interactions with neighboring particles are maintained. |
|
| 63 | A |
Objective 010 Response A is correct because in the bottom section of the curve, the substance is present only as a gas. This section of the graph corresponds to low pressure, where the particles occupy a larger volume. Response B is incorrect because the substance exists as a liquid in this section of the curve. This section of the curve represents an intermediate pressure and temperature range. Response C is incorrect because the substance will exist both as a solid and a liquid along this line. Response D is incorrect because at higher pressures, the substance will exist as a solid. Under conditions represented by the leftmost region of the graph, the substance exists entirely as a solid. |
|
| 64 | B |
Objective 010 Response B is correct because the ideal gas law PV = nRT is used to solve this problem. To find density, the number of moles, n, is replaced with . g grams divided by m molar mass This substitution is made and then the expression is rearranged to find . G grams divided by v density In this case, grams divided by density = . the product of p times m divided by the product of r times t The temperature is in kelvins and is 300 K. Pressure is 5.00 atm, as stated in the question, R is the ideal gas constant  0.0821 liters times atm per moles Kelvin
0.0821 liters times atm per moles Kelvin
 and the molar mass of oxygen is
.
32.0 grams per mole
Response A is incorrect and is obtained if the temperature is not converted to kelvins before being substituted into the expression. Response C is incorrect and is obtained if one fails to remember that oxygen is a diatomic molecule. A value of
16.0 grams per mole
for M is used to obtain the number given in this option. Response D is incorrect and is obtained by finding n using PV = nRT and then dividing by 20.0 L.
and the molar mass of oxygen is
.
32.0 grams per mole
Response A is incorrect and is obtained if the temperature is not converted to kelvins before being substituted into the expression. Response C is incorrect and is obtained if one fails to remember that oxygen is a diatomic molecule. A value of
16.0 grams per mole
for M is used to obtain the number given in this option. Response D is incorrect and is obtained by finding n using PV = nRT and then dividing by 20.0 L.
|
|
| 65 | C |
Objective 011 Correct Response C accurately reflects the polar nature of ethanol, which has an O H group that can hydrogen bond with polar water, and the nonpolar nature of ethane that has only London dispersion forces (interactions between induced-dipoles). Polar molecules have much stronger intermolecular forces than nonpolar molecules so that water will hydrogen bond with itself instead of interacting with ethane molecules. Response A is incorrect because ethanol is not ionic, and ethane is not a polar molecule. Response B is incorrect because ethane and ethanol have similar size. Furthermore, the interactions with ethanol are dominated by hydrogen bonding. Response D is incorrect because ethanol intermolecular forces are stronger than the ethane intermolecular forces, which is opposite of the statement. |
|
| 66 | C |
Objective 011 Response C is correct because ion-dipole interactions only occur between ions such as sodium ion ( N a + ) and polar molecules like water ( H 2 O ). Ion-dipole force is also the strongest interaction between N a + and H 2 O . Response A is incorrect because H 2 O does not contain any ions; the strongest interaction between H 2 O molecules is hydrogen bonding (very strong dipole-dipole forces). Response B is incorrect because it contains ions, but no polar molecules; the strongest interaction between the ions is ionic interactions. Response C is incorrect because octane and decane are neither ionic nor polar molecules; the strongest intermolecular forces between them is London dispersion forces, also called induced-dipole–induced-dipole interactions. |
|
| 67 | C |
Objective 011 Van der Waals forces between atoms and molecules come from the fluctuations of the electron clouds around atoms and molecules so that short-lived induced dipoles interact between nonpolar molecules. The more polarizable the atom or molecule is, the more readily the electron clouds fluctuate, and, therefore, the greater the induced-dipolar interactions (van der Waals forces) are (Correct Response C). Response A is incorrect because the forces tend to increase with atomic radii due to increased polarizability that is described in rationale for Response C. Response B is incorrect because decreasing surface area tends to decrease van der Waals forces because it reduces the region where induced-dipole interactions can occur. Response D is incorrect because branching of a molecule decreases extent of induced-dipole interactions for the molecules that are otherwise very similar (such as n-pentane and neopentane). |
|
| 68 | D |
Objective 011 Response D is correct because hydrogen bonds can occur in cases where hydrogen is covalently attached to fluorine, oxygen, or nitrogen. Hydrogen is covalently bound to nitrogen in ammonia, NH3. Responses A and B are incorrect since neither fluorine, oxygen, nor nitrogen is present in HCl or H2S. Response C is incorrect because hydrogen is covalently bound to carbon in CH3F. While fluorine is present in this compound, there is no hydrogen—fluorine bond. |
|
| 69 | D |
Objective 011 Response D is correct because nitrogen trichloride has a tetrahedral shape, and dipole-dipole forces are the primary intermolecular force. One of the four positions of the tetrahedron has a lone pair of electrons, and chlorine atoms occupy the other three positions. Because the molecule does not have a center of symmetry, it is polar, and dipole-dipole interactions are the predominant intermolecular force. Response A is incorrect because phosphorus pentachloride has a molecular geometry of trigonal bipyramidal, with no lone pairs. This molecule is, therefore, nonpolar and so will have London dispersion forces as the primary intermolecular force. Response B is incorrect for the same reason since carbon tetrachloride is tetrahedral, with no lone pairs of electrons. It is, therefore, nonpolar with London dispersion forces as the primary intermolecular force. Response C is incorrect for the same reason since beryllium chloride is a linear molecule, completely symmetrical with no lone pairs of electrons. It is, therefore, nonpolar with London dispersion forces as the primary intermolecular force. |
|
| 70 | B |
Objective 011 Response B is correct because the formula of methanol is C H 3 O H The formula of 1-hexanol is C H 3 C H 2 C H 2 C H 2 C H 2 C H 2 O H As can be observed from the formulas, the alkyl group that is nonpolar and contributes to insolubility is considerably longer in 1-hexanol than methanol. Because "like" dissolves "like" and because water is polar and the alkyl region is not, the alkyl region contributes to insolubility. Response A is incorrect because both formulas indicate the same number of hydroxyl groups. Response C is incorrect because 1-hexanol is considerably less polar due to the presence of the longer alkyl chain. Response D is incorrect because the only hydrogen atom that can interact via hydrogen bonding is on the hydroxyl group, and both molecules have the same number (1) of hydroxyl groups. |
|
| 71 | A |
Objective 012 Response A is correct because the equilibrium constant for this reaction can be expressed as K = Left bracket H2 S gaseous right bracket, times left bracket NH3 gaseous right bracket where the Left bracket, right bracket symbols represent concentration of that component in units of molarity (M). In this case, Left bracket H2 S gaseous right bracket = left bracket NH3 gaseous right bracket = 0.261 M, so K = 0.261 squared = 0.068. Response B is incorrect because it could be obtained only with equilibrium concentrations of 0.316 M. Response C is incorrect because it is off by a factor of about 10. Response D is incorrect because it is obtained by adding the concentrations of H2S and NH3. |
|
| 72 | D |
Objective 012 Response D is correct because the equilibrium constant for this reaction can be expressed as K sub e q = Left bracket h2 right bracket times Left bracket I 2 right bracket all over over Left bracket H I right bracket squared where the Left bracket, right bracket symbols represent equilibrium concentration of that component in units of molarity (M). In this case, Left bracket H2 right bracket = Left bracket I 2 right bracket = x so that Left bracket h2 right bracket times Left bracket I 2 right bracket = x squared and K sub e q = 0.02 Since 2 moles of H I are consumed for each mole of H2 produced, Left bracket H I right bracket equals the initial concentration of H I minus 2x right paren or 2 minus 2 x left paren Combining these relationships into the equilibrium constant equation gives 0.02 = . x squared divided by the square of the quantity 2 minus x Response A is incorrect because it fails to account for the 2 moles of H I consumed for each mole of H2 produced, it fails to account for H2 and I2 in the numerator (x instead of x squared ), and it fails to square the denominator as required by the equilibrium constant equation. Response B is incorrect because it fails to account for the 2 moles of H I consumed for each mole of H2 produced, and it fails to account for H2 and I2 in the numerator (x instead of x squared ). Response C is incorrect because it fails to account for the 2 moles of H I consumed for each mole of H2 produced, and it fails to square the denominator as required by the equilibrium constant equation. |
|
| 73 | C |
Objective 012 Response C is correct because for a generic equilibrium equation such as A plus B B yields C C plus D D the equilibrium constant equation is written as K sub e q = , the concentration of A to the a times the concentration of B to the b divided by the quantity of the concentration of C to the c times the concentration of D to the d where Left bracket, right bracket symbols represent concentrations of each component at equilibrium. At any moment in time, the reaction quotient, not necessarily at equilibrium, can also be written as Q = . the concentration of A to the a times the concentration of B to the b divided by the quantity of the concentration of C to the c times the concentration of D to the d Here the concentrations are not equilibrium concentrations. If Q = K sub e q , the system is at equilibrium. If Q is less than K sub e q (like in this case where Q = 0.74 vs. K sub e q = 14.5), there is excess reactant relative to product in the system, resulting in new product forming from reactants faster than new reactant is forming from products (Correct Response C). If Q is greater than K sub e q , there is excess product relative to reactant in the system, resulting in new reactant forming from products faster than new product is forming from reactants. Response A is incorrect because Q is not equal to K sub e q . Response B is incorrect because only products would require Q to be infinite (Q = 0.74). Response D is incorrect because Q would have to be greater than Keq (Q = 0.74 is less than K sub e q = 14.5). |
|
| 74 | C |
Objective 012 Response C is correct because a mixture of acetic acid ( H A c ) and sodium acetate ( N a A c ) will achieve equilibrium between H A c , acid ion (H+) and acetate ion ( A c minus ). The concentrations can be determined from the starting concentration and the equilibrium constant equation. The equilibrium constant is independent of the component concentrations (Correct Response C), although it is sensitive to changes in temperature. Response A is incorrect because adding HCl reduces the A c minus concentration and increases the H A c concentration, but does not change the equilibrium constant. Response B is incorrect because the buffer pH depends on the equilibrium constant, which changes with temperature. Response D is incorrect because the solution also has sodium ( N a + ) ions and H+ ions in addition to the H A c molecules and the A c minus ions. |
|
| 75 | D |
Objective 012 Response D is correct because changes in temperature result in a change in the equilibrium constant. Response A is incorrect because increasing the pressure will shift the equilibrium position to the left, but the equilibrium constant will be unaffected. Response B is incorrect because removing O2 as it is formed will shift the equilibrium position to the right, but the equilibrium constant will be unaffected. Response C is incorrect because adding a catalyst will increase the rate of the reaction, but the equilibrium constant will be unaffected. |
|
| 76 | A |
Objective 012 Response A is correct because by Le Chatelier's principle, removal of a product will cause the system to respond in such a way as to form more product in order to restore equilibrium. In this case, restoring equilibrium will require a shift to the right. Response B is incorrect because increasing the pressure will favor the side with the smaller number of moles of gas. In this case, it will cause a shift to the left because there are 7 moles of gas on the right and 2 moles of gas on the left (water is in the liquid state). Response C is incorrect because the reaction given here is endothermic (the enthalpy change is positive). Heat is treated as a reactant in such cases, so its removal will cause a shift to the left. Response D is incorrect because the water is in the liquid phase. Substances in the liquid phase are not considered to have an effect on the equilibrium position. |
|
| 77 | D |
Objective 013 Response D is correct because two components will react only if they collide with enough kinetic energy to overcome the activation energy of the reaction and with the right orientation to each other so that the reaction pathway is followed. Increasing the kinetic energy of the reacting system will increase the likelihood that the collision has enough kinetic energy. Increasing the number of collisions increases the likelihood that a collision with the right orientation occurs. The number of collisions can be increased by increasing the number of one or both reacting particles present in the solution for a unit of volume—in other words by increasing its concentration (Correct Response D). Response A is incorrect because reducing the temperature reduces the number collisions with enough kinetic energy for the reaction to occur, thus slowing the reaction. Response B is incorrect because the size of powder particles before dissolving has no impact on the kinetics of the dissolved particles. Particle size matters only if a solid surface participates in the reaction. Response C is incorrect because rate is defined as change in concentration (amount per unit volume) divided by change in time; therefore, while increasing the volume will increase the total number of collisions, the number of collisions per unit volume will not change. |
|
| 78 | B |
Objective 013 Response B is correct because in a chemical reaction, reactant and product concentration stop changing, as appears to happen around 25 minutes, when the rates of the forward and reverse reactions are equal. This also indicates equilibrium is achieved. Response A is incorrect because concentration of both the reactant and product continue to change with time at 5 minutes, so equilibrium is not achieved. Response C is incorrect because no information is available to evaluate the rate law for the forward or reverse reactions. Response D is incorrect because no data is available to evaluate the activation energy of either the forward or reverse reaction. |
|
| 79 | C |
Objective 013 Response C is correct because in this reversible reaction, the rate of forward reaction will decrease, and the rate of the reverse reaction will increase over time until they converge at equilibrium. Once at equilibrium, changing temperature, changing pressure, or adding a catalyst will change the reaction rate, but only adding a catalyst will change the forward and reverse reactions equally by reducing the activation energy for both directions. Response A is incorrect because increasing the temperature favors reactants formation ( delta H is less than 0 ). Therefore, the reverse reaction rate will increase and the forward reaction rate will decrease, and then they will reconverge until a new equilibrium is achieved. Response B is incorrect because increasing pressure will favor product formation so that the forward reaction rate will increase and the reverse reaction rate will decrease, and then they will reconverge until a new equilibrium is achieved. Response D is incorrect because this is a gas phase reaction, so there are no solid reactive components to surface modify. |
|
| 80 | C |
Objective 013 Response C is correct because a comparison of the results from experiments 1 and 4 indicates that when the concentration of A is doubled, the reaction rate is also doubled. This result indicates that the reaction is first order in A2, meaning that the concentration of A2 A 2 open paren open bracket A 2 close bracket close paren is raised to the first power in the rate expression. A comparison of the results from experiments 1 and 2 indicates that when the concentration of B is tripled, the reaction rate is also tripled. This result indicates that the reaction is also first order in B. Response A is incorrect because this expression implies that the reaction rate does not depend on the concentration of B (which is clearly not the case, based on the data). Response B is incorrect because the ratio of concentration to reaction rate is 1 to 1 in both A and B. Response D is also incorrect because the reaction rate doubles as the concentration of A is doubled (i.e., a 1 to 1 ratio). |
|
| 81 | B | Objective 013 Response B is correct because the first step in determining the reaction rate for the specified concentration of H2O2 is to determine the rate constant, k. This is accomplished using the experimental data provided and the rate equation for the reaction: rate = k times the quantity h 2 o 2 to the enth power . The value of n is determined by evaluating the experimental data. Data from experiments 1 and 2 show that when the the quantity h 2 o 2 is doubled, the initial rate doubles. This corresponds to a value of 1 for n. The values for initial rate and initial the quantity h 2 o 2 for any given experiment can be obtained from the table and substituted into the equation k = . initial rate divided by the quantity h 2 o 2 This calculation yields a value of 1.06 times 10 to the negative 3 minutes to the negative 1 for k. The calculated value of k along with the specified the quantity h 2 o 2 can then be substituted into the rate equation for the reaction, rate = k equals initial rate divided by the quantity h 2 o 2 . Multiplying 1.06 times 10 to the negative 3 minutes to the negative 1 by 6.50 times 10 to the negative 1 mole gives a reaction rate of 6.89 times 10 to the negative 4 Moles per liter per minute Response A is incorrect and is obtained using a k value of 9.78 times 10 to the negative 5 minutes to the negative 1 in the rate equation. Response C is incorrect and is obtained using a k value of 7.06 times 10 to the negative 2 minutes to the negative 1 in the rate equation. Response D is incorrect and is obtained by omitting k from the rate equation, using rate = the quantity h 2 o 2 . |
|
| 82 | B | Objective 013 Response B is correct because k times the concentration of N O 2 squared corresponds to the slow step of the reaction. The slow step of the reaction is the one that most strongly affects the rate law. Step 2 and the overall equation are not included when writing the rate law. The "2" in the rate law comes from the fact that there is a coefficient of 2 in front of N O 2 in Step 1. Response A is incorrect because the expression for rate must include the reactants in the rate-limiting (slow) step. Response C is incorrect because this expression is based on Step 2, which is the fast step, not the slow step. Response D is incorrect because this expression for the rate law includes some of the reactants in the overall reaction, but not the reactants in the slow step. |
|
| 83 | B | Objective 014 Response B is correct because percent error is a measure of accuracy, which is defined as how close the result is to the true value. Reducing percent error improves accuracy. Reducing factors that cause deviation from the true value improve accuracy and reduce percent error. If heat is lost from the calorimeter to the environment, the magnitude of the experimentally measured enthalpy change will be less than the true value due to heat loss; therefore, reducing the heat loss improves accuracy and will reduce percent error. Response A is incorrect because fewer data points will reduce the precision, but it will not impact the accuracy. Response C is incorrect because increasing thermometer precision will increase the enthalpy measurement precision, but it will not impact the accuracy. Response D is incorrect because increasing the amount of ethanol in the burner will increase the magnitude of the temperature change, but will not necessarily improve the accuracy of the measurement because the heat loss will also likely increase with the increased temperature change. |
|
| 84 | C | Objective 014 Response C is correct because since pentanol has more C—C and C—H bonds to break to convert it to C O 2 and water, its heat of combustion is more negative. Therefore, more heat is evolved in the complete reaction of an equal mole amount of pentanol as ethanol, and, therefore, more energy is released. Response A is incorrect because there should be a greater temperature increase with pentanol than will ethanol. Response B is incorrect because percent error is related to the heat lost to the environment. Heat loss will likely increase as more heat is produced; therefore, percent error may also increase. Response D is incorrect because there is no information to indicate if the pentanol will burn for a longer time or for a shorter time. |
|
| 85 | D | Objective 014 Response D is correct because basically, entropy increases with the "disorder" of the system; therefore, a liquid of the same material as a solid is greater entropy than the solid, a gas of the same material is greater entropy than both liquid and solid, and a solution of a material is greater entropy than a solid of the same material. Increasing number of types of particles also increases entropy, and increasing number of gas particles increases entropy. For Correct Response D, the reactant has a solid and 6 moles of a gas, whereas the product contains 12 moles of gas and, therefore, is greater entropy than the reactants. Response A is incorrect because solid water (product) has lower entropy than liquid water (reactant). Response B is incorrect because there is the same number of gas particles for product and reactant; however, since the reactants are two different gases, the entropy of mixing of the two reactant gases would be greater than the entropy of the pure product gas. Response C is incorrect because the Ag+ and Cl minus aqueous ions are greater entropy than the solid product AgCl. |
|
| 86 | A | Objective 014 Response A is correct because the amount of heat required to raise the temperature of 50.0 g of copper from 25.0°C to 100°C is calculated as q equals m s delta T so q = 50.0 grams times 0.385 Joules per gram kelvin times 75 kelvins The temperature difference is calculated by first converting the temperatures to Kelvins and then finding the difference (373 K minus 298 K). Response B is incorrect and can be calculated as 50.0 grams times 0.385 Joules per gram kelvin times 100 kelvins Response C is incorrect and can be calculated as 50.0 grams times 0.385 Joules per gram kelvin times 348 kelvins Response D is incorrect and can be calculated as 50.0 grams times 0.385 Joules per gram kelvin times 373 kelvins |
|
| 87 | B | Objective 014 Response B is correct because the specific heat of the metal may be calculated by assuming that heat gained by the water was lost by the metal. If we let q represent the amount of heat that was transferred from the water in the calorimeter to the metal, the mathematical expression that results is m sub w s sub w delta T equals m sub m s sub m delta T where m represents mass, s represents specific heat, and Delta T is the temperature change. The subscripts in the formula are w (indicating water) and m (indicating the metal). Since heat is calculated as m s Delta T the left side of the expression represents the amount of heat lost by the water, and the right side represents the amount of heat gained by the metal. Using values for these variables from this example, the expression becomes 100 grams times 4 point 1 8 4 joules per gram Kelvin times 5 Kelvin equals 100 grams times s sub m times the difference of 353 point 1 5 Kelvin and 298 point 1 5 Kelvin Solving for the specific heat of the metal gives 0.38 J/(g•K), as given in Correct Response B. The temperature change could have been determined without first converting to kelvins because a change of 1 degree Celsius is equivalent to a change of 1 kelvin. Response A is incorrect because 0.26 J/(g•K) is obtained using Delta T of 80 K on the right side of the equation. Response C is incorrect because 1.52 J/(g•K) is obtained using 4 point 1 8 4 joules per gram Kelvin times 100 grams divided by 273 point 1 5 Kelvin Response D is incorrect because 1.90 J/(g•K) is obtained using 4 point 1 8 4 joules per gram Kelvin times 100 grams divided by 220 Kelvin |
|
| 88 | C | Objective 014 Response C is correct because the total heat change, q, in the constant pressure calorimeter, measured in joules, is calculated as m s Delta T , where m is the mass, s is the specific heat, and Delta T is the change in temperature. Since 12.3 g of K C l O 3 was dissolved in 200.0 g of water, the total mass is 212.3 g. The value of s, the specific heat of the solution, is given in the problem as 4.184 J/(g•K). The change in temperature is 4.95 K, since the calorimeter started with a temperature of 25.00°C and dropped to 20.05°C at the end of the experiment and because a change of 1 degree Celsius is equivalent to a change of 1 kelvin. Once q is calculated, it is divided by 1,000 to convert joules to kilojoules and is then divided by the number of moles of K C l O 3 , which is 0.100. Response A is incorrect and was calculated using m s Delta T , but the mass of the water was left out of this expression and a value of 12.3 g was used for mass. Further, the number of moles of K C l O 3 was not considered. Response B is incorrect and was calculated by converting incorrectly from joules to kilojoules and omitting the step of dividing by moles of K C l O 3 . Response D is incorrect; 168 kJ/mol can be obtained by using incorrect values for mass (200.0 g) and Delta T (5.00 K) and dividing the product of ms by Delta T . |
|
| 89 | A | Objective 015 Response A is correct because for a reaction to be spontaneous, the free energy change ( Delta G ) of the reaction must be negative ( Delta G is less than 0). Using the free energy equation Delta G = Delta H minus T Delta S , the reaction will be spontaneous if T Delta S is greater than Delta H . A reaction is not spontaneous ( Delta G is greater than 0) at any temperatures if Delta H is greater than 0 and Delta S is less than 0. In contrast, a reaction will be spontaneous ( Delta G is less than 0) at all temperatures (Correct Response A) if Delta H is less than 0 and Delta S is greater than 0 (as in this problem). For any other conditions, there will be a temperature threshold between nonspontaneous and spontaneous reaction. Response B is incorrect because it sets a temperature threshold for a spontaneous reaction when there is no threshold, since Delta H is less than 0 and Delta S is greater than 0. Response C is incorrect because Delta G is less than 0 at all temperatures, so the reaction is spontaneous at all temperatures. Response D is incorrect because it sets a temperature threshold for a spontaneous reaction above where it is not spontaneous when there is no threshold because Delta H is less than 0 and Delta S is greater than 0. |
|
| 90 | B | Objective 015 Response B is correct because the free energy equation can be represented as Delta G = standard delta G + R T natural log of Q At equilibrium, Delta G = 0, and Q = K, giving Delta G = 0 = standard delta G + R T natural log of K Rearranging gives standard delta G = negative R T natural log of K Since K much greater than R T natural log of K is positive making standard delta G less than 0, meaning that equilibrium favors products (Correct Response B). Response A is incorrect because no information is available to indicate the temperature dependence of K. Response C is incorrect because equilibrium does not favor reactants, and no information is available to indicate the temperature dependence of K. Response D is incorrect because standard delta G less than 0 and, therefore, the equilibrium does not favor reactants. |
|
| 91 | C | Objective 015 Response C is correct because the enthalpy of a reaction ( Delta H) can be approximately equated to the bond enthalpies of reactants and products as delta H equals the sum of D sub bonds broken minus the sum of D sub bonds formed where D represents bond enthalpy of reactants (bonds broken) and products (bonds formed). In this problem, Delta H = negative 9 kiloJoules per mole = D sub H to H +D sub I to I minus 2D H to I Rearranging to solve for D sub I to I gives D sub I to I = negative 9 kiloJoules per mole minus D sub H to H + 2 D sub H to I, or D sub I to I = open bracket negative 9 minus 432 + 2 open parens 295 close parens close bracket kJ/mol = 149 kJ/mol. Response A is incorrect because it only accounts for the reaction enthalpy and the bond enthalpy of 1 mol of H to I, and it does not account for H to H bond enthalpy. Furthermore, the result is negative, which is not possible for a stable bond. Response B is incorrect because it only accounts for the reaction enthalpy, and it does not account for the H to H or the 2H to I bond energy. Furthermore, the result is negative, which is not possible for a stable bond. Response D is incorrect because it only accounts for the reaction enthalpy and the bond enthalpy of 1 mol of H to I, and it does not account for H to H bond energy. |
|
| 92 | B | Objective 015 Response B is correct because the enthalpy of formation is calculated by summing the bond enthalpies for the bonds that are broken and then subtracting the enthalpy for the bonds that are formed. The numbers in the table are given in units per mole, and this is taken into account in the calculations. The correct equation is 1 half(498 kJ/mol + 243 kJ/mol) minus 2x = 105 kJ/mol, where x is the bond enthalpy for an O to Cl bond. The coefficient of 2 in front of x is needed because there are two O to Cl bonds in O C l 2 . Solving for x, we obtain the value given in Correct Response B. Response A is incorrect and was calculated using the value of 138 kJ/mol for the bond enthalpy of O2. This is incorrect because O2 has a double bond in the correct Lewis structure. Response C is incorrect and was calculated by omitting the coefficient of 1 half in front of 498 kJ/mol in the expression above. This coefficient is necessary because it is present in the balanced chemical equation. Response D is incorrect and was calculated without the coefficient of 2 in front of the x in the expression given above. |
|
| 93 | B | Objective 015 Response B is correct because the energy required to break the O2 bond is equal to the energy released when an O2 bond forms from two oxygen atoms. Therefore, 499 kJ/mol = the sum of the heats of formation of the products (two individual O atoms) minus the heat of formation of the reactant (O2). The equation is as follows: 499 kJ/mol = 2 times (x) + 0, where x equals the heat of formation of the individual oxygen atoms and zero is the heat of formation of O2, a substance in its elemental state. Response A is incorrect and is the heat of formation of diatomic oxygen. Response C is incorrect and is the bond energy for oxygen gas. Response D is incorrect and is twice the bond energy for oxygen gas. |
|
| 94 | A | Objective 015 Response A is correct because reactions are spontaneous when Gibbs free energy is negative. Gibbs free energy is equal to standard delta H minus temperature times standard delta S where the standard delta H term represents the enthalpy change, the standard delta S term represents the entropy change, and T represents temperature. If T is high, the second term in the expression for Gibbs free energy will be greater than the first term, and the overall Gibbs free energy will be negative. Response B is incorrect because the enthalpy term is negative and has a greater magnitude than the entropy term. Gibbs free energy for this reaction will be negative at a range of temperatures. Response C is incorrect because the Gibbs free energy for this expression will be positive at a range of temperatures, since the entropy term is negative. Response D is incorrect because this reaction will have a positive Gibbs free energy at high temperatures. |
|
| 95 | D | Objective 015 Response D is correct because the change in enthalpy for a reaction is obtained by taking the potential energy of the products and subtracting the potential energy of the reactants. In this case, d represents the potential energy of the products and a represents the potential energy of the reactants. Response A is incorrect and is equal to the energy of the reactants with a minus sign in front. Response B is incorrect and is equal to the energy of the reactants. Response C is incorrect and represents the energy of the reactants minus the energy of the products. |
|
| 96 | B | Objective 016 Response B is correct because the electrochemical potential can be determined from the standard reduction potential for the reduction half-reaction C L 2 pipe C L minus minus the standard reduction potential for the oxidation half-cell A L pipe A L 3 plus which is positive 1 point 3 6 minus negative 1 point 6 6 equals 3 point 0 2 volts Response A is incorrect because the voltage calculated is negative 0 point 1 3 minus negative 2 point 9 3 equals 2 point 8 0 volts Response C is incorrect because the voltage calculated is 1 point 5 0 minus 0 point 8 5 equals 0 point 6 5 volts Response D is incorrect because the voltage calculated is 0 point 1 3 minus negative 2 point 3 7 equals 2 point 5 0 volts |
|
| 97 | B | Objective 016 Response B is correct because the plot of the cell potential against log copper ( 2 ) concentration shows that point #2 is below the trendline while the other three points are very close to the trendline. By repeating trial 2, the measured result is expected to be closer to the trendline, resulting in a trendline fit that is closer to all the measured data points and, therefore, more precise. Response A is incorrect because there is no evidence that the voltmeter is working incorrectly. Furthermore, changing voltmeters will likely impact accuracy, but not precision if both voltmeters have the same measurement precision. Response C is incorrect because changing the stock solution used to prepare the test solutions may change the starting point of the cell potential measurement, but it will not change the precision. Response D is incorrect because more trials below 0.005 M is an ambiguous solution. It may increase precision, but it would not be as effective as repeating trial 2. |
|
| 98 | D | Objective 016 Response D is correct because the positive slope of the plot shows directly that cell potential increases with increasing log of copper ( 2 ) concentration, or conversely that cell potential decreases with decreasing log of copper ( 2 ) concentration meaning it also decreases with decreasing concentration of the cathode half-cell. Response A is incorrect because cell potential does not show proportionality to solution concentration, but rather a linear relationship to the log of the copper ( 2 ) concentration. Response B is incorrect because, even though the Nernst equation E sub cell equals E standard sub cell minus the quantity R times T over N times F times the natural log of Q where Q equals the concentration of Z N 2 plus divided by the concentration of C U 2 plus shows a relationship between cell potential and Zn2+ concentration, it is not supported by the data since variation of the anode half-cell concentration was not studied. Response C is incorrect because moles of electrons transferred depends on the cell potential, which varies in this analysis. |
|
| 99 | A | Objective 016 Response A is correct because in this galvanic cell, the standard reduction potential of C u is greater than Zn. Therefore, reduction occurs at C u electrode, called the cathode ( 2 ), and oxidation occurs at the Zn electrode, called the anode ( 1 ). To maintain the charge balance in the respective solutions, S O 4 2 minus travels from the salt bridge into the Z N 2 plus slash S O 4 2 minus solution, and the N a + from the salt bridge travels to the C U 2 + slash S O 4 2 minus solution. Also, the "excess" electrons generated by oxidation Zn travels as a current from the anode to the cathode ( 3 ). Response B is incorrect because it incorrectly identifies the electron flow direction. Response C is incorrect because it incorrectly identifies the anode and cathode. Response D is incorrect because it incorrectly identifies the anode and cathode, and it incorrectly identifies the electron flow direction. |
|
| 100 | A | Objective 016 Response A is correct because the standard cell potential or electromotive force ( e m f ) can be calculated using the following equation: E standard sub cell equals E standard sub cathode minus E standard sub anode. The E standard values used for both the cathode and the anode in the equation are the standard reduction potentials of the electrodes shown in the table. The net cell potential is positive in this case and equals 0.84 V Negative 0 point 3 4 volts minus negative 1 point 1 8 volts The cell given in Correct Response A is the only option in which the net potential is positive, which indicates the cell is spontaneous at standard conditions. A positive net cell potential corresponds to a negative value for Gibbs free energy, which indicates the formation of products is favored. Response B is incorrect because the net cell potential is Negative 1.98 volts Negative 1 point 1 8 volts minus negative 0 point 8 0 volts Response C is incorrect because the net potential is negative 3.51 volts Negative 2 point 7 1 volts minus negative 0 point 8 0 volts Response D is incorrect because the net cell potential is negative 0.20 volts Negative 2 point 9 1 volts minus negative 2 point 7 1 volts |
|
| Total Correct: | Review your results against the test objectives. | ||
Open Responses, Sample Responses, and Analyses
| Question Number |
Your Response Read about how your responses are scored and how to evaluate your practice responses |
||
|---|---|---|---|
| 101 |
Open Response Item Assignment #1 For each assignment, you may type your written response on the assigned topic in the box provided. Note: The actual test allows you to handwrite your responses on separate response sheets to be scanned for upload to the test. For this practice test, you may handwrite each response on 1–2 sheets of paper. |
||
| First Sample Weak Response |
First Sample Weak Response to Open-Response Item Assignment #1 The kinetic molecular theory represents gas as empty space with numerous very small particles that move and collide frequently. Our atmosphere is an example. If the gas is in a container like a balloon, it behaves according to the gas laws. Particles of air at room temperature move fast compared with the same particles at low temperatures in the freezer. The volumes will be different at different temperatures: higher temperature will have a bigger volume; lower temperature will have a smaller volume. The mass of the air in the balloon doesn't change. Teachers can ask students to measure the circumference of a balloon at different temperatures. This experiment will give the same conclusion as above. |
||
| First Weak Response Analysis |
Analysis of First Weak Response to Open-Response Item Assignment #1 This is an example of a weak response because it is characterized by the following: Purpose: This response only partially achieves the purpose of the prompt. Key scientific concepts are limited, such as defining gas as "empty space with numerous very small particles that move and collide." There is no representative diagram or graph. The classroom activity does not "extend" what is already being investigated in the prompt itself. Subject Matter Knowledge: The kinetic molecular theory of a gas is partially explained. The response demonstrates some knowledge about the composition of a gas according to the theory but does not go far enough in consideration of the kinetic energy of the particles. Little new scientific vocabulary is utilized other than particles of air, different temperature, and circumference. Support: The gas law is mentioned but poorly explained. The experiment is not sufficiently developed nor is it supported with a formula or a diagram. Consequently, the results, the different circumferences of the balloon, are not clear. Rationale: The response shows limited understanding of the basic concepts of kinetic molecular theory. The ability to reason through the application of kinetic molecular theory to the phenomenon is lacking. |
||
| Second Sample Weak Response |
Second Sample Weak Response to Open-Response Item Assignment #1 The kinetic molecular theory describes how the particles that make up solids, liquids, and gases behave. The molecules of all three are in motion and have kinetic energy. The motion is greater in higher temperatures because the molecules move faster and have increased kinetic energy as the temperature goes up. As the temperature goes down, the molecules lose some kinetic energy and slow down. When an inflated balloon is put in the freezer, the balloon, and the air inside it, cool down. The gas molecules lose energy, slow down, and get closer to each other causing the balloon to deflate. The volume of the balloon gets smaller after being cooled showing the gas law that explains the relationship between temperature and volume. The teacher could propose an investigation where the inflated balloon is put in a warmer environment like an oven. Students could observe the changes the balloon undergoes to reinforce the gas law about temperature and volume. |
||
| Second Weak Response Analysis |
Analysis of Second Weak Response to Open-Response Item Assignment #1 This is an example of a weak response because it is characterized by the following: Purpose: The purpose of the assignment is partially achieved. Although all three bullets are attempted, they are not well described and supported. The key scientific concepts of molecular theory to gas laws are simplistically explained and lack a graph, formula, or diagram that shows the relationship between temperature and the movement of gas in a balloon. The proposed investigation is equally limited. Subject Matter Knowledge: The partial explanation of the kinetic molecular theory is an insufficient scientific explanation of kinetic energy and the gas laws to support it. The "gas law" is alluded to but not clearly stated using scientific vocabulary and formulas. Little new scientific vocabulary is utilized other than prompt language. Support: No diagram, formula, or graph to model the situation is provided, limiting the support to simplistic explanations: "the molecules lose some kinetic energy and slow down." Rationale: The response shows some limited understanding of the concepts, but lacks the depth of support of a stronger response. The proposed investigation is a poorly reasoned extension of the investigation of putting a balloon in a freezer by putting a balloon in an oven, the results of which are obvious and require little scientific thought. |
||
| First Sample Strong Response |
First Sample Strong Response to Open-Response Item Assignment #1 With a finite amount of a gas in a specific volume and a variation of temperature keeping the pressure constant, there is a variance in the volume. This is because at lower temperatures the molecules in the balloon will move at slower speeds. The molecules will then bounce off the walls of the balloon at a lower rate. This will cause the volume of the balloon to decrease. The mass of the balloon will not change because no gas is escaping from the balloon. The same amount of material is in the balloon at both temperatures. This is an example of Charles' Law that states: the volume of a gas varies directly with its temperature measured in K. (See equation below.) A graphical relationship can be demonstrated by measuring the volumes at different temperatures of a variety of balloons inflated with different amounts of air. A chemistry teacher could extend this investigation for students to see the effects of the gas laws using large plastic syringes with locking valves attached. Students could predict the direction of movement of the syringe plunger when the syringe is submerged in water baths of various temperatures, then collect data by using any graduations on the syringe to note specific volume changes. The gas volume could be graphed over the range of the water baths. This will demonstrate Charles' Law in real life situations. V sub 1 divided by T sub 1 equals V sub 2 divided by T sub 2 |
||
| First Strong Response Analysis |
Analysis of First Strong Response to Open-Response Item Assignment #1 This is an example of a strong response because it is characterized by the following: Purpose: This strong response demonstrates a fully achieved purpose. The three bullets are fully addressed with key scientific concepts such as the use of Charles' Law to describe the relationship between temperature and volume. A representative graph is described. The investigation in the classroom is clearly described with appropriate detail. This response reflects thorough knowledge and strong understanding of the topic. Subject Matter Knowledge: The molecular movement of the gas in the balloon is accurately described and the connection to Charles' Law is clearly stated. Changing temperature and volume are identified as variables and demonstrate an understanding of Charles' Law and knowledge of scientific investigation. Support: The experiment described would enable students to gather data about temperature and volume and show it in a graphical relationship. Rationale: The practical examples, the soda at different temperatures, extend the investigation. The clear explanation, the use of scientific vocabulary, and the inclusion of Charles' Law with a formula demonstrate a comprehensive understanding of the required concepts. |
||
| Second Sample Strong Response |
Second Sample Strong Response to Open-Response Item Assignment #1 The physical properties of any gas are defined by four variables: pressure(P), temperature(T), volume(V), and number of moles(n). The Gas Laws define the relationships between these four variables. The kinetic molecular theory describes the behavior of gases at the particle level. The molecules of gas are in constant random motion, and they collide with each other and the walls of the container. The collisions are elastic so that when two molecules collide, they change their directions, but their total kinetic energy is conserved. The molecules exert no attractive forces on each other or on the walls of the container. The average kinetic energy of the gas molecules is directly proportional to the temperature. Kinetic energy is the energy an object has due to its motion (see first equation below). As the temperature of a gas rises, the average velocity of the molecules will increase. Conversely, as the temperature of a gas decreases, the average velocity of the molecules will decrease. When an inflated balloon is placed in the freezer, the balloon and its contents will cool. As the temperature of the gas molecules decreases, their average kinetic energy will also decrease. They will move with less velocity and strike the walls of the container with less momentum. The balloon will appear partially deflated as a result of fewer collisions acting on the interior wall. The mass of the balloon and its contents will be the same before and after going into the freezer. No gas has escaped, but the molecules are closer together and more condensed due to the lower temperature. Charles' Law states that the volume of a gas varies directly with its temperature (see second equation below). In this case as the temperature drops, the volume decreases and the flexible balloon shrinks or deflates. A practical example of Charles' Law and how different inflated objects shrink as the temperature decreases would be a car's tires. When the outside temperature drops, the tires may appear deflated. It becomes important to check the tire pressure and to add air as needed. For the engineering practice of extending investigations the teacher could introduce Boyle's Law which describes the relationship between pressure and volume: P equals 1 over V and ask students to predict the changes in a gas that is being compressed by using a syringe or piston to decrease its volume. There is no temperature change in this example, so there would be no change in the speed with which the particles move, but the space occupied will be smaller resulting in an increase in the frequency of collisions with the walls leading to an increase in the pressure of the gas. Students would conclude that the pressure of the gas becomes greater as the volume of the gas becomes smaller.
|
||
| Second Strong Response Analysis |
Analysis of Second Strong Response to Open-Response Item Assignment #1 This is an example of a strong response because it is characterized by the following: Purpose: The response demonstrates a strong score because the assignment is fully achieved. The three bullets are fully addressed. There is a clear and elaborated explanation of the kinetic molecular theory and gas law with relevant, scientific vocabulary. Two formulas, kinetic energy and Charles' Law are accurately provided. The extended investigation introduces a new law to enhance students' understanding of kinetic molecular theory, key concepts, and their application. Subject Matter Knowledge: The concept of kinetic molecular theory is thoroughly explained along with the four basic properties of gases: pressure, temperature, volume, and number of moles. These concepts help to explain that the balloon deflates in a cold environment without losing gas. Charles' Law is stated and its formula is provided. The relationship of Charles' Law to the kinetic molecular theory is pointed out. The kinetic molecular theory's explanation and formula illustrate the Gas Law in a clear and understandable manner. Support: Finally, a second gas law is introduced, Boyle's Law. By applying kinetic molecular theory to a new situation involving no temperature change, but a volume change, the students could again use their knowledge of kinetic molecular theory to explain how volume and pressure are related. The addition of a new situation reinforces the investigation and expands the knowledge base of the students. Rationale: The response demonstrates a thorough and comprehensive understanding of the subject matter. The response includes detailed explanations, scientific vocabulary, and formulas, as well as definitions of kinetic energy, Charles' Law, and Boyle's Law. The given example of the balloon in cold and room temperatures is extended with the practical example of the changes in car tires in the same environments. |
||
| 102 |
Open-Response Item Assignment #2 For each assignment, you may type your written response on the assigned topic in the box provided. Note: The actual test allows you to handwrite your responses on separate response sheets to be scanned for upload to the test. For this practice test, you may handwrite each response on 1–2 sheets of paper. |
||
| First Sample Weak Response |
First Sample Weak Response to Open-Response Item Assignment #2 If different substances are dissolved in water, the freezing point should be lowered. The different substances that will be dissolved are salt, sugar and oil. The chemistry teacher will make a 0.50 molal solution of each substance and attempt to freeze a small amount by placing them in an ice bath with rock salt. They should freeze at a temperature below 0 degrees C. They may not all freeze at the same temperature. Why? This is a topic for discussion. |
||
| First Weak Response Analysis |
Analysis of First Weak Response to Open-Response Item Assignment #2 This is an example of a weak response because it is characterized by the following: Purpose: The response demonstrates a limited understanding of the subject matter. Three of the four bullets are attempted: there is a testable claim, a partially described investigation, and a proposal for class discussion. The third bullet is not satisfied as there is no evidence that supports or refutes the claim that the freezing point should be lowered when different substances are dissolved in water. Subject Matter Knowledge: A testable claim is stated: "If different substances are dissolved in water, the freezing point should be lowered." The investigation described mentions the variables of salt, sugar, and oil, but does not include the amounts of each substance that are dissolved. There is no control group, and the procedure is poorly explained because it lacks the clarity of scientific investigations. The data to be collected is not clearly described; however, temperature is mentioned. There is no mention of how the data could be used to get evidence to support or refute the tested claim. There is scant scientific vocabulary utilized: "0.50 molal solution" and "below 0°C" are examples. Support: With no data collected, there lacks evidence that either supports or refutes the claim. There is nothing additional suggested for bullet four other than a proposal for a discussion session to make sense of why the substances may have different freezing temperatures. Rationale: The response shows some understanding, such as the freezing temperature of water will be lowered with the addition of solutes, but lacks the depth of support needed for even an adequate score. There is no suggested investigation as to how a chemistry teacher could use the engineering practice of "planning and carrying out investigations" to help students make sense of the effects of solutes on the freezing point of water. The response demonstrates a limited, poorly reasoned understanding of the topic. |
||
| Second Sample Weak Response |
Second Sample Weak Response to Open-Response Item Assignment #2 The freezing point of water is affected by adding salts (solutes) as when road salt or products like 'Ice Melt' are used on sidewalks to melt ice and keep it from refreezing. The salt lowers the freezing point of the water, and this helps to keep the ice melted even when temperatures are around or below freezing, 32 degrees F. To show this effect, small containers of water with different amounts of salt could be put in the freezer along with a container of water with no salt. A thermometer could be put into each, and observations could be made every few minutes. The time it takes for each to freeze would be recorded as well as the temperature and any changes in the contents. The results should show which container freezes the quickest and which ones take longer. Students could test to see what other substances could be added to water to bring about a similar effect. |
||
| Second Weak Response Analysis |
Analysis of Second Weak Response to Open-Response Item Assignment #2 This is an example of a weak response because it is characterized by the following: Purpose: The response demonstrates a limited achievement of the purpose. All four bullets are attempted. There is a testable claim that the freezing point of water is affected by adding salts (solutes). The investigation described lacks specificity such as the amount of water in the containers and the different amounts of salts used. The description of data collection is vague: observations could be made every few minutes. The culminating investigation to help students make sense of the effects of solutes on the freezing point of water is equally vague: students could test to see what other substances could be added to water to bring about a similar effect. Subject Matter Knowledge: The response shows some basic understanding of how the freezing point can be affected by salts from everyday experiences: using Ice Melt on frozen surfaces. Scientific language is not included in the description of the investigation. The response proposes containers with different amounts of salt, but this is an inappropriate manner to discuss variables since the response lacks specific amounts of salt per container. There is a control mentioned in the container of water without salt; however, the container is not labeled as a control nor are the amounts of water per container fully described. The explanation of the collected data is insufficient as there is no mention of specific time and temperature. The lack of clarity and specificity of explanation demonstrates a limited understanding of the scientific method. Support: The procedure lacks details and consequently the expected result is not clearly demonstrated. The results should show which container freezes the quickest and which ones take longer. The response should demonstrate a clear understanding of the results such as the results will show that the container with the largest amount of salt will freeze at the lowest temperature. Equally as vague is the description of the follow-up activity to make sense of the topic: "Students could test to see what other substances could be added to water to bring about a similar effect." Rationale: Because the response is very general and lacks a depth of support, there is a demonstration of limited and poorly reasoned understanding of the topic. Using the word "should" rather than "will" demonstrates limited understanding. The overall approach is very general in that the effect of adding solutes to water is described, but lacks the depth of development to demonstrate a comprehensive understanding of the topic. |
||
| First Sample Strong Response |
First Sample Strong Response to Open-Response Item Assignment #2 By dissolving different solutes in water, the freezing point of water is altered. Water normally freezes at a temperature of 0 degrees C. By dissolving a solute in water, the freezing point will be lowered. By dissolving different amounts of different solutes in a certain amount of water, we can measure how much the temperature will be lowered. The amount of lowering of the freezing point depends solely on the molality of the resulting solution. The molality depends upon the number of ions or molecules that are dissolved in water. Students will observe a demonstration showing freezing point depression of a water solution, before designing an investigation based on solutions of either sodium chloride, magnesium chloride, or sucrose. Students will choose a substance and be challenged to design an experiment to predict the freezing point of an aqueous solution of the substance, of unknown concentration. Students will be provided with rock salt and ice to freeze their solutions and will be able to use a variety of glassware in their experimental designs. Teacher feedback will encourage student groups to consider using smaller volumes to decrease freezing time. Graphing the freezing point depression against the molality would result in a straight line with the slope being the freezing point depression constant, 1.86 in the case of water. Students will hypothesize that using solutes can effectively lower the freezing point, causing a mixture to freeze when put in an environment containing salt added to ice. Students will put flavoring, cream and sugar into a small zip-lock bag and will put crushed ice into a gallon sized zip-lock bag (control). One group of students will add one-half cup of salt (variable) to the ice. The small bags of ingredients will be placed into the larger ice bags. Students will shake the bags of ice, checking the small bag of ingredients at 2-minute intervals to determine when the mixture is frozen. Then the small bag will be carefully removed and students will taste the ice cream they have made. Ice cream should form in the variable group where the freezing point was lowered. |
||
| First Strong Response Analysis |
Analysis of First Strong Response to Open-Response Item Assignment #2 This is an example of a strong response because it is characterized by the following: Purpose: The response demonstrates a strong score because the assignment is fully achieved. The four bullets are fully addressed and answered with substantial subject matter knowledge such as the need for specific solutions of N A C L, M G C L 2 and sucrose, the use of a formula to calculate the freezing point of water (1.86 m) and a detailed description of the graph offered as support. The relevant and scientific vocabulary includes molality, ions, and molecules. Other examples of lowered freezing point include the use of rock salt on winter ice and the addition of rock salt to ice in an ice cream maker. Subject Matter Knowledge: A testable claim—"by dissolving a solute in water, the freezing point will be lowered"—is stated followed by a detailed description of an investigation that could be done to test the claim. Specific scientific information is included such as the use of solutions of sodium chloride, magnesium chloride, or sucrose. The explanation of a graph, where m is the molality of the solution and 1.86 is the freezing point depression constant, shows strong subject matter knowledge. Support: Strong support includes the variables and data to be collected and a graph of freezing point depression vs molality. The explanation of how the slope would be a straight line representing the freezing point depression constant shows good understanding of the mathematical relationship between concentration and freezing point depression. The expected outcome that would support the claim is stated as predicted temperature drops for each solution, and also by the trend of the graph. Rationale: The response is thoroughly answered and demonstrates comprehensive understanding of the subject matter. The real-world application of making ice cream shows clear and accurate reasoning about the freezing point of water and how it is affected by solutes. |
||
| Second Sample Strong Response |
Second Sample Strong Response to Open-Response Item Assignment #2 The freezing point of water can be lowered by dissolving various materials into it. Students will take different amounts of different substances, dissolve them in a known quantity of water, and then measure the temperature at which the solution will freeze. Knowing the molality of the solution students can predict what the freezing point should be, and can compare it with what is determined experimentally. Prepare 0.5, 1.0, and 1.5 molal solutions of glucose, N A C L and M G C L 2 . Take 10 ml of each solution and place them in clean test tubes. Place the test tubes into an ice bath into which you have added rock salt. This will lower the temperature of the ice below zero. Place a thermometer into each of the test tubes. Watch the solutions and observe at what temperature crystals begin to form. This will be the freezing point of that solution. Calculate what the freezing point should be by the formula: delta T sub f equals k sub f m The change in temperature equals the freezing point constant K sub f times the molality of the solution. K sub f water = 1.86 Collected data, the freezing points of the various solutions, will demonstrate that the higher the molality of the solutions the more the freezing point will be lowered. If the molality is too high, the solution will not freeze in this lab with the stated concentrations. This concept of lowering the freezing point is used in a variety of everyday uses from melting ice to keeping a car engine at a running temperature. When salt is sprinkled on ice, the freezing point of the ice is lowered so that it will melt. When ethylene glycol is added to water in the radiator of a car, the water will not freeze while in the car. These are just a couple of the practical uses in which the concept of freezing point depression is used in everyday life. |
||
| Second Strong Response Analysis |
Analysis of Second Strong Response to Open-Response Item Assignment #2 This is an example of a strong response because it is characterized by the following: Purpose: This strong response demonstrates a fully achieved application of the prompt. There is a testable scientific claim proven with a detailed scientific investigation, and the collected data from the investigation is thoroughly discussed. The practical solutions mentioned in the extended investigation—salt sprinkled on ice and the addition of glycol to a car engine—help students make sense of the effects of solutes on the freezing point of water. Subject Matter Knowledge: A stated testable claim—"the freezing point of water can be lowered by dissolving various materials into it"—is followed by a detailed description of a procedure that could be done to test the claim. Specific steps and clear instructions are given for the procedure. The variables—0.5, 1.0, and 1.5 molal solutions of glucose; N A C L and M G C L 2 data to be collected; the freezing points of various solutions—are indicated, and a formula to determine the freezing point by calculation is also given: "delta T sub f equals k sub f m where K sub f water = 1.86." This shows understanding of the mathematical relationship of concentration and freezing point depression. Support: The expected outcome that supports the claim is stated: "the higher the molality of the solutions the more the freezing point will be lowered." The outcome of the experiment to real world applications demonstrates the relevance of the concept as it is used in everyday life. Rationale: The response is thoroughly answered with scientific vocabulary and a clear understanding of the scientific method. Reasoning is sound with the demonstration of variables in the explanation of the investigation, and both the mathematical relationship and the conclusion are clear and understandable. The response demonstrates comprehensive understanding of the subject matter. |
||
| Review the Performance Characteristics and Score Scale for Written Performance Assignments. | |||
Multiple Choice Question
Practice Test Evaluation Chart
In the evaluation chart that follows, the multiple-choice questions are arranged in numerical order and by test objective. Check your responses against the correct responses provided to determine how many questions within each objective you answered correctly.
Subarea 1 : Matter and Its Interactions: Periodic Properties
Objective 0001: Apply knowledge of atomic and subatomic structure and the principles of quantum theory.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 1 | D | |
| 2 | A | |
| 3 | A | |
| 4 | B | |
| 5 | A | |
| 6 | C | |
| 7 | D | |
| 8 | B | |
| 9 | B |
out of 9
Objective 0002: Apply knowledge of periodic properties and the organization of the periodic table.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 10 | D | |
| 11 | A | |
| 12 | D | |
| 13 | C | |
| 14 | D | |
| 15 | A |
out of 6
Subarea 1 (Objectives 0001–0002) Total out of 15
Subarea 2 : Matter and Its Interactions: Chemical Structure and Reactions
Objective 0003: Apply knowledge of the nomenclature and structure of inorganic and organic compounds.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 16 | B | |
| 17 | C | |
| 18 | A |
out of 3
Objective 0004: Apply knowledge of chemical bonding and the relationship between bond type and the properties of substances.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 19 | A | |
| 20 | A | |
| 21 | A | |
| 22 | A | |
| 23 | B | |
| 24 | D |
out of 6
Objective 0005: Apply knowledge of physical and chemical properties and physical and chemical changes.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 25 | D | |
| 26 | A | |
| 27 | D | |
| 28 | D | |
| 29 | A | |
| 30 | B | |
| 31 | A |
out of 7
Objective 0006: Apply knowledge of the types and characteristics of chemical reactions.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 32 | D | |
| 33 | B | |
| 34 | D | |
| 35 | D | |
| 36 | A |
out of 5
Objective 0007: Apply knowledge of the quantitative relationships expressed in chemical equations.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 37 | C | |
| 38 | C | |
| 39 | B | |
| 40 | C | |
| 41 | A | |
| 42 | A |
out of 6
Subarea 2 (Objectives 0003–0007) Total out of 27
Subarea 3 : Matter and Its Interactions: Substances, Mixtures, and Solutions
Objective 0008: Apply knowledge of the mass relationships in chemical substances.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 43 | B | |
| 44 | A | |
| 45 | C | |
| 46 | C | |
| 47 | A | |
| 48 | C |
out of 6
Objective 0009: Analyze the properties of solutions.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 49 | C | |
| 50 | D | |
| 51 | C | |
| 52 | D | |
| 53 | C | |
| 54 | B | |
| 55 | D | |
| 56 | A | |
| 57 | B |
out of 9
Subarea 3 (Objectives 0008–0009) Total out of 15
Subarea 4 : Motion and Stability: Forces and Interactions
Objective 0010: Apply knowledge of the kinetic molecular theory, the nature of phase changes, and the gas laws.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 58 | D | |
| 59 | D | |
| 60 | C | |
| 61 | C | |
| 62 | B | |
| 63 | A | |
| 64 | B |
out of 7
Objective 0011: Apply knowledge of the connection between molecular structure and forces between particles.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 65 | C | |
| 66 | C | |
| 67 | C | |
| 68 | D | |
| 69 | D | |
| 70 | B |
out of 6
Objective 0012: Apply knowledge of the principles of chemical equilibrium.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 71 | A | |
| 72 | D | |
| 73 | C | |
| 74 | C | |
| 75 | D | |
| 76 | A |
out of 6
Subarea 4 (Objectives 0010–0012) Total out of 19
Subarea 5 : Energy in Chemical Systems
Objective 0013: Apply knowledge of the factors that affect reaction rates and methods of measuring reaction rates.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 77 | D | |
| 78 | B | |
| 79 | C | |
| 80 | C | |
| 81 | B | |
| 82 | B |
out of 6
Objective 0014: Apply knowledge of the principles of thermodynamics and calorimetry.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 83 | B | |
| 84 | C | |
| 85 | D | |
| 86 | A | |
| 87 | B | |
| 88 | C |
out of 6
Objective 0015: Apply knowledge of the energy relationships in chemical bonding and chemical reactions.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 89 | A | |
| 90 | B | |
| 91 | C | |
| 92 | B | |
| 93 | B | |
| 94 | A | |
| 95 | D |
out of 7
Objective 0016: Apply knowledge of oxidation-reduction reactions to electrochemistry.
| Question Number | Your Response | Correct Response |
|---|---|---|
| 96 | B | |
| 97 | B | |
| 98 | D | |
| 99 | A | |
| 100 | A |
out of 5
Subarea 5 (Objectives 0013–0016) Total out of 24
Practice Test Score Calculation
The practice test score calculation is provided so that you may better gauge your performance and degree of readiness to take an MTEL test at an operational administration. Although the results of this practice test may be used as one indicator of potential strengths and weaknesses in your knowledge of the content on the official test, it is not possible to predict precisely how you might score on an official MTEL test.
The Sample Responses and Analyses for the open-response items may help you determine whether your responses are more similar to the strong or weak samples. The Scoring Rubric can also assist in estimating a score for your open responses. You may also wish to ask a mentor or teacher to help evaluate your responses to the open-response questions prior to calculating your total estimated score.
How to Calculate Your Practice Test Score
Review the directions in the sample below and then use the blank practice test score calculation worksheet to calculate your estimated score.
Multiple-Choice Section
| Enter the total number of multiple-choice questions you answered correctly: | 63 | ||
| Use Table 1 below to convert that number to the score and write your score in Box A: | A: | 192 |
Open-Response Section
| Enter the number of points (1 to 4) for your first open-response question: | 3 | ||
| Enter the number of points (1 to 4) for your second open-response question: | 3 | ||
| Add those two numbers (Number of open-response question points): | 6 | ||
| Use Table 2 below to convert that number to the score and write your score in Box B: | B: | 50 |
Total Practice Test Score (Estimated MTEL Score)
| Add the numbers in Boxes A and B for an estimate of your MTEL score: | A + B = | 242 |
Practice Test Score Calculation Worksheet: Chemistry (67)
Table 1:
| Number of Multiple-Choice Questions Correct | Estimated MTEL Score |
|---|---|
| 0 to 25 | 140 |
| 26 to 30 | 147 |
| 31 to 35 | 153 |
| 36 to 40 | 160 |
| 41 to 45 | 166 |
| 46 to 50 | 173 |
| 51 to 55 | 179 |
| 56 to 60 | 186 |
| 61 to 65 | 192 |
| 66 to 70 | 198 |
| 71 to 75 | 205 |
| 76 to 80 | 211 |
| 81 to 85 | 218 |
| 86 to 90 | 224 |
| 91 to 95 | 231 |
| 96 to 100 | 237 |
Table 2:
| Number of Open-Response Question Points | Estimated MTEL Score |
|---|---|
| 2 | 31 |
| 3 | 36 |
| 4 | 41 |
| 5 | 46 |
| 6 | 50 |
| 7 | 55 |
| 8 | 60 |
Use the form below to calculate your estimated practice test score.
Multiple-Choice Section
| Enter the total number of multiple-choice questions you answered correctly: | |||
| Use Table 1 above to convert that number to the score and write your score in Box A: | A: |
Open-Response Section
| Enter the number of points (1 to 4) for your first open-response question: | |||
| Enter the number of points (1 to 4) for your second open-response question: | |||
| Add those two numbers (Number of open-response question points): | |||
| Use Table 2 above to convert that number to the score and write your score in Box B: | B: |
Total Practice Test Score (Estimated MTEL Score)
| Add the numbers in Boxes A and B for an estimate of your MTEL score: | A + B = |